Co-Cyprindiol 2000/35 Coated Tablets
SUMMARY OF PRODUCT CHARACTERISTICS
"W This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions. See section 4.8 for how to report adverse reactions.
1 NAME OF THE MEDICINAL PRODUCT
Co-cyprindiol 2000/35 Coated Tablets
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each tablet contains 2000 micrograms cyproterone acetate and 35 micrograms of ethinylestradiol.
Excipients with known effect:
Each tablet contains;
29.115mg lactose monohydrate 19.637mg sucrose
For the full list of excipients, see section 6.1
3 PHARMACEUTICAL FORM
Coated tablets
Round, biconvex, yellow sugar-coated tablets with a 5.7mm nominal diameter.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Treatment of moderate to severe acne related to androgen-sensitivity (with or without seborrhoea) and/or hirsutism, in women of reproductive age.
For the treatment of acne, Co-cyprindiol Tablets should only be used after topical therapy or systemic antibiotic treatments have failed.
Since Co-cyprindiol Tablets is also a hormonal contraceptive, it should not be used in combination with other hormonal contraceptives (see section 4.3).
4.2 Posology and method of administration
This product inhibits ovulation and thereby prevents conception. Patients who are using this product should not therefore use an additional hormonal contraceptive, as this will expose the patient to an excessive dose of hormones and is not necessary for effective contraception.
First treatment course:
One tablet daily for 21 days, starting on the first day of the menstrual cycle (the first day of menstruation counting as Day 1).
Subsequent courses:
Each subsequent course is started 7 tablet-free days after the preceding course.
Complete remission of acne is to be expected in nearly all cases, often within a few months, but in particularly severe cases treatment for longer periods may be necessary before the full benefit is seen. It is recommended that treatment be withdrawn when the acne or hirsutism has completely resolved. Repeat courses of Co-cyprindiol Tablets may be given if the condition recurs.
When the contraceptive action of Co-cyprindiol Tablets is also to be employed, it is essential that the above instructions be rigidly adhered to. Should bleeding fail to occur during the tablet-free interval, the possibility of pregnancy must be excluded before the next pack is started.
When changing from an oral contraceptive and relying on the contraceptive action of Co-cyprindiol Tablets, the instructions given below should be followed:
Changing from 21-day combined oral contraceptives:
The first Co-cyprindiol Tablet should be taken on the first day immediately after the end of the previous oral contraceptive course. Additional contraceptive precautions are not required.
Changing from a combined Every Day Pill (28 day tablets):
The first Co-cyprindiol Tablet should be taken the day after taking the last active tablet from the Every Day Pill pack. Additional contraceptive precautions are not then required.
Changing from a progestogen-only pill (POP):
The first Co-cyprindiol Tablet should be taken on the first day of bleeding, even if a POP has already been taken on that day. Additional contraceptive precautions are not then required. The remaining progestogen-only pills should be discarded.
Post-partum and post-abortum use:
After pregnancy, Co-cyprindiol Tablets can be started 21 days after a vaginal delivery, provided the patient is fully ambulant and there are no puerperal complications. Additional contraceptive precautions will be required for the first 7 days of pill taking. Since the first post-partum ovulation may precede the first bleeding, another method of contraception should be used in the interval between childbirth and the first course of tablets.
After a first-trimester abortion, Co-cyprindiol Tablets may be started immediately and no additional contraceptive precautions are required.
Duration of use
Time to relieve of symptoms is at least three months. The need to continue treatment should be evaluated periodically by the treating physician.
Special circumstances requiring additional contraception:
Incorrect administration:
A single delayed tablet should be taken as soon as possible, and if this is within 12 hours of the correct time, contraceptive protection is maintained. With longer delays, additional contraception is needed. Only the most recently delayed tablet should be taken, earlier missed tablets being omitted. Additional non-hormonal methods of contraception (except the rhythm or temperature methods) should be used for the next 7 days, while the next 7 tablets are being taken. Also, if tablet(s) have been missed during the last 7 days of a pack, there should be no break before the next pack is started. In this situation, a withdrawal bleed should not be expected until the end of the second pack. Some breakthrough bleeding may occur on tablet taking days but this is not clinically significant. If the patient does not have a withdrawal bleed during the tablet-free interval following the end of the second pack, the possibility of pregnancy must be ruled out before starting the next pack.
Gastro-intestinal upset:
Vomiting or diarrhoea may reduce the efficacy of oral contraceptives by preventing full absorption. Tablet-taking from the current pack should be continued and additional non-hormonal methods of contraception (except the rhythm or temperature methods) should be used during the gastro-intestinal upset, and for 7 days following the upset. If these 7 days overrun the end of a pack, the next pack should be started without a break. In this situation, a withdrawal bleed should not be expected until the end of the second pack. If the patient does not have a withdrawal bleed during this period the possibility of pregnancy must be ruled out before starting the next pack. Other methods of contraception should be considered if the gastro-intestinal disorder is likely to be prolonged.
4.3 Contraindications
1. Pregnancy or lactation
2. Severe disturbances of liver function, jaundice or persistent itching during a previous pregnancy, Dubin-Johnson syndrome, Rotor syndrome, previous or existing liver tumours.
3. Personal or family history of confirmed, idiopathic venous thromboembolism (VTE) (where a family history refers to VTE in a sibling or parent at a relatively early age).
4. Concomitant use with another hormonal contraceptive (see section 4.1)
5. Venous thrombosis present or in history (deep venous thrombosis, pulmonary embolism)
6. Arterial thrombosis present or in history (e.g. myocardial infarction) or prodromal conditions (e.g. angina pectoris and transient ischaemic attack)
7. Present or history of cerebrovascular accident
8. The presence of a severe or multiple risk factor(s) for venous or arterial thrombosis (see section 4.4) such as:
• diabetes mellitus with vascular symptoms
• severe hypertension
• severe dyslipoproteinaemia
9. Hereditary or acquired predisposition for venous or arterial thrombosis, such as activated protein C (APC) resistance, antithrombin-III-deficiency, protein C deficiency, protein S deficiency, hyperhomocysteinaemia and antiphospholipid-antibodies (anticardiolipin-antibodies, lupus anticoagulant).
10. Sickle-cell anaemia.
11. Mammary or endometrial carcinoma, or a history of these conditions.
12. History of herpes gestationis.
13. Deterioration of otosclerosis during pregnancy.
14. Undiagnosed abnormal vaginal bleeding.
15. Hypersensitivity to the active substances or to any of the excipients of Co-cyprindiol Tablets.
16. Pancreatitis or a history thereof if associated with severe hyper triglyceridemia.
17. History of migraine with focal neurological symptoms.
4.4 Special warnings and precautions for use
Co-cyprindiol Tablets are composed of the progestogen cyproterone acetate and the oestrogen ethinylestradiol and is administered for 21 days of a monthly cycle. It therefore has a similar composition to that of a combined oral contraceptive (COC).
Duration of use
Time to relief of symptoms is at least three months. The need to continue treatment should be evaluated periodically by the treating physician (see section 4.2).
If any of the conditions/risk factors mentioned below is present, the benefits of the use of Co-cyprindiol Tablets should be weighed against the possible risks for each individual woman and discussed with the woman before she decides to start using Co-cyprindiol Tablets. In the event of aggravation, exacerbation or first appearance of any of these conditions or risk factors, the woman should contact her physician. The physician should then decide on whether the use of Co-cyprindiol Tablets should be discontinued.
Warnings:
Like many other steroids, the combination of cyproterone acetate and ethinylestradiol when given in very high doses and for the majority of the animal’s life-span, has been found to cause an increase in the incidence of tumours, including carcinoma, in the liver of rats. The relevance of this finding to humans is unknown.
In rare cases benign and, in even rarer cases, malignant liver tumours leading in isolated cases to life-threatening intra-abdominal haemorrhage have been observed after the use of hormonal substances such as those contained in Co-cyprindiol Tablets. If severe upper abdominal complaints, liver enlargement or signs of intra-abdominal haemorrhage occur, a liver tumour should be included in the differential diagnosis.
Animal studies have revealed that feminisation of male foetuses may occur if cyproterone acetate is administered during the phase of embryogenesis at which differentiation of the external genitalia occurs. Although the results of these tests are not necessarily relevant to man, the possibility must be considered that administration of Co-Cyprindiol Tablets to women after the
45th day of pregnancy could cause feminisation of male foetuses. It follows
from this that pregnancy is an absolute contraindication for treatment with Co-
cyprindiol Tablets, and must be excluded before treatment is begun.
Circulatory disorders
• The use of Co-cyprindiol Tablets carries an increased risk of venous thromboembolism (VTE) compared with no use. The excess risk of VTE is highest during the first year a woman starts Co-cyprindiol Tablets or when restarting or switching after a pill-free interval of at least a month. Venous thromboembolism can be fatal in 1-2% of cases.
• Epidemiological studies have shown that the incidence of VTE is 1.5 to 2 times higher in users of Co-cyprindiol Tablets than in users of levonorgestrel-containing combined oral contraceptives (COCs) and may be similar to the risk for desogestrel / gestodene / drospirenone-containing COCs.
• The user group of Co-cyprindiol Tablets is likely to include patients that may have an inherently increased cardiovascular risk such as that associated with polycystic ovarian syndrome.
• Epidemiological studies have also associated the use of hormonal contraceptive with an increased risk for arterial (myocardial infarction, transient ischaemic attack) thromboembolism.
• Extremely rarely, thrombosis has been reported to occur in other blood vessels, e.g. hepatic, mesenteric, renal, cerebral or retinal veins and arteries, in hormonal contraceptive users.
• Symptoms of venous or arterial thrombosis or of a cerebrovascular accident can include: unusual unilateral leg pain and / or swelling; sudden severe pain in the chest, whether or not it radiates to the left arm; sudden breathlessness; sudden onset of coughing; any unusual, severe, prolonged headache; sudden partial or complete loss of vision; diplopia; slurred speech or aphasia; vertigo; collapse with or without focal seizure; weakness or very marked numbness suddenly affecting one side or one part of the body; motor disturbances; ‘acute’ abdomen
• The risk of venous thromboembolic events increases with: o increasing age;
o smoking (with heavier smoking and increasing age the risk further increases, especially in women over 35 years of age. Women over 35 years of age should be strongly advised not to smoke if they wish to use Co-cyprindiol Tablets);
o a positive family history (i.e. venous thromboembolism ever in a sibling or parent at a relatively early age). If a hereditary predisposition is suspected, the woman should be referred to a specialist for advice before deciding about any hormonal contraceptive use;
o prolonged immobilisation, major surgery, any surgery to the legs, or major trauma. In these situations it is advisable to discontinue use (in the case of elective surgery at least four weeks in advance) and not to resume until two weeks after complete remobilisation. Antithrombotic treatment should be considered if the use of Co-cyprindiol Tablets has not been discontinued in advance.
o obesity (body mass index over 30 kg/m ).
• The risk of arterial thromboembolic complications or of a cerebrovascular accident increases with:
o increasing age;
o smoking (with heavier smoking and increasing age the risk further increases, especially in women over 35 years of age. Women over 35 years of age should be strongly advised not to smoke if they wish to use Co-cyprindiol Tablets);
o dyslipoproteinemia; o obesity (body mass index over 30 kg/m2); o hypertension; o migraine;
o valvular heart disease; o atrial fibrillation;
o a positive family history (arterial thrombosis ever in a sibling or parent at a relatively early age). If a hereditary predisposition is suspected, the woman should be referred to a specialist for advice before deciding about any hormonal contraceptive use.
• Other medical conditions, which have been associated with adverse circulatory events, include diabetes mellitus, systemic lupus erythematosus, hemolytic uraemic syndrome, chronic inflammatory bowel disease (e.g. Crohn's disease or ulcerative colitis) and sickle cell disease.
• The increased risk of thromboembolism in the puerperium must be considered (for information on ‘Pregnancy and lactation’ see section 4.6).
• An increase in frequency or severity of migraine during use of Co-cyprindiol Tablets (which may be prodromal of a cerebrovascular event) may be a reason for immediate discontinuation of Co-cyprindiol Tablets.
Women using Co-cyprindiol Tablets should be specifically pointed out to contact their physician in case of possible symptoms of thrombosis. In case of suspected or confirmed thrombosis, Co-cyprindiol Tablets use should be discontinued. Adequate contraception should be initiated because of the teratogenicity of anti-coagulant therapy (coumarins). There is no consensus about the possible role of varicose veins and superficial thrombophlebitis in venous thromboembolism.
Biochemical factors indicating a hereditary or acquired predisposition to venous or arterial thrombosis include APC resistance, hyperhomocysteinemia, antithrombin III deficiency, protein C deficiency, protein S deficiency, phospholipid antibodies (cardiolipin antibodies, lupus anticoagulant).
Tumours
Numerous epidemiological studies have been reported on the risks of ovarian, endometrial, cervical and breast cancer in women using combined oral contraceptives. The evidence is clear that combined oral contraceptives offer substantial protection against both ovarian and endometrial cancer.
An increased risk of cervical cancer in long-term users of combined oral contraceptives has been reported in some studies, but there continues to be
controversy about the extent to which this is attributable to the confounding effects of sexual behaviour and other factors.
A meta-analysis from 54 epidemiological studies reported that there is a slightly increased relative risk (RR = 1.24) of having breast cancer diagnosed in women who are currently using combined oral contraceptives (COCs). The observed pattern of increased risk may be due to an earlier diagnosis of breast cancer in COC users, the biological effects of COCs or a combination of both. The additional breast cancers diagnosed in current users of COCs or in women who have used COCs in the last ten years are more likely to be localised to the breast than those in women who never used COCs.
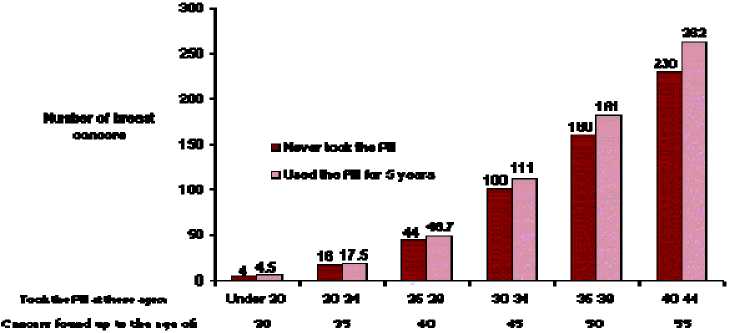
Breast cancer is rare among women under 40 years of age whether or not they take COCs. Whilst this background risk increases with age, the excess number of breast cancer diagnosed in current and recent COC users is small in relation to the overall risk of breast cancer (see bar chart).
The most important risk factor for breast cancer in COC users is the age women discontinue the COC; the older the age at stopping, the more breast cancers are diagnosed. Duration of use is less important and the excess risk gradually disappears during the course of the 10 years after stopping COC use such that by 10 years there appears to be no excess risk.
The possible increase in risk of breast cancer should be discussed with the user and weighed against the benefits of COCs as they offer substantial protection against the risk of developing certain other cancers (e.g. ovarian and endometrial cancer).
Estimated cumulative numbers of breast cancers per 10,000 women diagnosed in 5 years of use and up to 10 years after stopping COCs, compared with numbers of breast cancers diagnosed in 10,000 women who had never used COCs.
The possibility cannot be ruled out that certain chronic diseases may occasionally deteriorate during the use of Co-cyprindiol Tablets (see Precautions).
Other conditions
Women suffering from hypertriglyceridemia or having this disease in family anamnesis can exhibit an increased risk of pancreatitis in the course of use of combined oral contraceptives.
Although small increases in blood pressure have been reported in many women taking COCs, clinically relevant increases are rare. However, if a sustained clinically significant hypertension develops during the use of a COC then it is prudent for the physician to withdraw the COC and treat the hypertension. Where considered appropriate, COC use may be resumed if normotensive values can be achieved with antihypertensive therapy.
The following conditions have been reported to occur or deteriorate with both pregnancy and COC use, but the evidence of an association with COC use is inconclusive: Jaundice and/or pruritus related to cholestasis; gallstone formation; porphyria; systemic lupus erythematosus; hemolytic uremic syndrome; Sydenham’s chorea; herpes gestationis; otosclerosis-related hearing loss.
In women with hereditary angioedema exogenous oestrogens may induce or exacerbate symptoms of angioedema.
Acute or chronic disturbances of liver function may necessitate the discontinuation if COC use until markers of liver function return to normal. Recurrence of cholestatic jaundice which occurred first during pregnancy or previous use of sex steroids necessitates the discontinuation of COC.
Although COCs may have an effect on peripheral insulin resistance and glucose tolerance, there is no evidence for a need to alter the therapeutic regimen in diabetics using low-dose COCs (containing < 0.05mg ethinylestradiol). However, diabetic women should be carefully observed while taking COCs.
Crohn’s disease and ulcerative colitis have been associated with COC use. Chloasma may occasionally occur, especially in women with a history of chloasma gravidarum. Women with a tendency to chloasma should avoid exposure to the sun or ultraviolet radiation whilst taking COCs.
Women should be advised that oral contraceptives do not protect against HIV infections (AIDS) and other sexually transmitted diseases.
Reduced efficacy:
The efficacy of COCs may be reduced in the event of e.g. missed tablets, gastro-intestinal disturbances or concomitant medication.
Reduced cycle control:
With all COCs, irregular bleeding (spotting or breakthrough bleeding) may occur, especially during the first months of use.
In some women withdrawal bleeding may not occur during the tablet-free interval. It the COC has been taken according to the directions described in section 4.2 it is unlikely that the woman is pregnant. However, if the COC has not been taken according to these directions prior to the first missed withdrawal bleed or if two withdrawal bleeds are missed, pregnancy must be ruled out before COC use is continued.
Reasons for stopping Co-cyprindiol Tablets immediately:
1. Occurrence, or exacerbation of migraines, headaches or unusually frequent or severe headaches.
2. Sudden disturbances of vision or hearing or other perceptual disorders.
3. First signs of thrombophlebitis or thromboembolic symptoms (e.g. unusual pains in or swelling of the leg(s), stabbing pains on breathing or coughing for no apparent reason). Feeling of pain and tightness in the chest.
4. Six weeks before an elective major operation (e.g. abdominal, orthopaedic), any surgery to the legs, medical treatment for varicose veins or prolonged immobilisation, e.g. after accidents or surgery. Co-cyprindiol Tablets should not be restarted until 2 weeks after full ambulation. In case of emergency surgery, thrombotic prophylaxis is usually indicated e.g. subcutaneous heparin.
5. Onset of jaundice, hepatitis, itching of the whole body.
6. Increase in epileptic seizures.
7. Significant rise in blood pressure.
8. Onset of severe depression.
9. Severe upper abdominal pain or liver enlargement.
10. Clear worsening of conditions known to deteriorate during use of hormonal contraception or during pregnancy.
11. Pregnancy is a reason for stopping immediately because it has been suggested by some investigations that oral contraceptives taken in early pregnancy may slightly increase the risk of foetal malformations. Other investigations have failed to support these findings. The possibility therefore cannot be excluded, but it is certain that if a risk exists at all, it is small.
Precautions:
Assessment of women prior to starting oral contraceptives (and at regular intervals thereafter) should include a personal and family medical history of each woman. Physical examination should be guided by this and by the contraindications (section 4.3) and warnings (section 4.4) for this product. The frequency and nature of these assessments should be based upon relevant guidelines and should be adapted to the individual woman, but should include measurement of blood pressure and, if judged appropriate by the clinician, breast, abdominal and pelvic examination including cervical cytology.
The following conditions require strict medical supervision during medication with oral contraceptives. Deterioration or first appearance of any of these conditions may indicate that Co-cyprindiol Tablets should be discontinued:
Diabetes mellitus or a tendency towards diabetes mellitus (e.g. unexplained glycosuria). hypertension, varicose veins, a history of phlebitis, otosclerosis, multiple sclerosis, epilepsy, porphyria, tetany, disturbed liver function, Sydenham's chorea, renal dysfunction, family history of clotting disorders, obesity, family history of breast cancer and patient history of benign breast disease, history of clinical depression, systemic lupus erythematosus, uterine fibroids, an intolerance to contact lenses, migraine, gall-stones, cardiovascular diseases, chloasma, asthma, or any disease that is prone to worsen during pregnancy.
Patients with a history of depression or any condition mentioned above should be monitored during treatment with Co-cyprindiol Tablets.
If Co-cyprindiol Tablets are discontinued, other methods of contraception should be introduced if needed.
The use of ultraviolet lamps, for the treatment of acne, or prolonged exposure to sunlight, increases the risk of the deterioration of chloasma.
Some women may experience amenorrhoea or oligomenorrhoea after discontinuation of Co-cyprindiol Tablets, especially when these conditions existed prior to use. Women should be informed of this possibility.
Patients with rare hereditary problems of fructose intolerance, glucose-galactose malabsorption or sucrase-isomaltase insufficiency should not take this medicine.
Patients with rare hereditary problems of galactose intolerance, the Lapp lactase deficiency or glucose-galactose malabsorption should not take this medicine.
4.5 Interaction with other medicinal products and other forms of interaction
Hepatic enzyme inducers such as oxcarbazapine, topiramate, felbamate, ritonavir, products containing St. John’s wort, barbiturates, primidone, phenobarbitone, phenytoin, phenylbutazone, rifampicin, carbamazepine and griseofulvin can impair the contraceptive efficacy of Co-cyprindiol Tablets. For women receiving long-term therapy with hepatic enzyme inducers, another method of contraception should be used.
HIV protease (e.g. ritonavir) and non-nucleoside reverse transcriptase inhibitors (e.g. nevirapine, and combinations of them, have been reported to potentially affect hepatic metabolism.
Some clinical reports suggest that enterohepatic circulation of oestrogens may decrease when certain antibiotic agents are given.
The use of antibiotics may also reduce the contraceptive efficacy of Co-cyprindiol Tablets.
Women receiving short courses of enzyme inducers and broad spectrum antibiotics should take additional, non-hormonal (except rhythm or temperature method) contraceptive precautions during the time of concurrent medication and for 7 days afterwards. If these 7 days overrun the end of a pack, the next pack should be started without a break. In this situation, a withdrawal bleed should not be expected until the end of the second pack. If the patient does not have a withdrawal bleed during the tablet-free interval following the end of the second pack, the possibility of pregnancy must be ruled out before resuming with the next pack.
Oral contraceptives may affect the metabolism of certain other drugs. Accordingly, plasma and tissue concentrations may either increase (e.g. ciclosporin) or decrease (e.g. lamotrigine).
The possibility cannot be ruled out that oral tetracyclines, if used in conjunction with Co-cyprindiol Tablets may reduce its contraceptive efficacy. When drugs of these classes are being taken it is, therefore, advisable to use additional non-hormonal methods of contraception (except the rhythm or temperature methods) since an extremely high degree of protection must be provided when Co-cyprindiol Tablets is being taken. With rifampicin, additional contraceptive precautions should be continued for 4 weeks after treatment stops, even if only a short course was administered.
The requirement for oral antidiabetics or insulin can change as a result of the effect on glucose tolerance.
The herbal remedy St John's wort (Hypericum perforatum) should not be taken concomitantly with Co-cyprindiol Tablets as this could potentially lead to a loss of contraceptive effect.
4.6 Fertility, pregnancy and lactation Pregnancy
Pregnancy is an absolute contraindication for treatment with Co-cyprindiol Tablets and must be excluded before such treatment is begun.
Animal studies have revealed that feminisation of male foetuses may occur if cyproterone acetate is administered during the phase of embryogenesis when differentiation of the external genitalia occurs. Although these findings are not necessarily relevant to man, pregnancy is an absolute contraindication for treatment with Co-cyprindiol Tablets, and must be excluded before treatment is begun.
Breastfeeding
Co-cyprindiol is contraindicated during lactation. Cyproterone acetate passes into the milk of the nursing mother. About 0.2% of a dose given to the mother is passed to a newborn from the milk, which corresponds to a dose of about 1pg/kg. During breast feeding, about 0.2% of ethinylestradiol from the daily dose given to the mother might be passed to the newborn from the milk.
4.7 Effects on ability to drive and use machines
None known
4.8 Undesirable effects
There is an increased risk of venous thromboembolism for all women who use this product. For more information see section 4.4.
Psychiatric disorders
Not known: Depressive moods, changes in libido
Nervous system disorders Not known: Headache
Eye disorders
Not known: Poor tolerance of contact lenses
Vascular disorders Rare: Thromboembolism
Gastrointestinal disorders
Not known: Abdominal pain, nausea, vomiting
Reproductive system and breast disorders Not known: Breast tenderness
Investigations
Not known: Weight increase, weight decrease
In predisposed women, use of Co-cyprindiol Tablets can sometimes cause chloasma which is exacerbated by exposure to sunlight. Such women should avoid prolonged exposure to sunlight.
The following serious adverse events have been reported in women using Co-cyprindiol Tablets, which are discussed in section 4.4:
• Venous thromboembolic disorders
• Arterial thromboembolic disorders
Menstrual changes:
1. Reduction of menstrual flow - this is not abnormal and is to be expected in some patients.
2. Missed menstruation - occasionally withdrawal bleeding may not occur at all. If the tablets have been taken correctly, pregnancy is unlikely. Should bleeding fail to occur during the tablet-free interval the possibility of pregnancy must be excluded before the next pack is started.
3. Intermenstrual bleeding - "spotting" or heavier "breakthrough bleeding" can sometimes occur during tablet taking, especially in the first few cycles, but normally cease spontaneously. Co-cyprindiol Tablets should therefore, be continued even if irregular bleeding occurs. If irregular bleeding is persistent, appropriate diagnostic measures to exclude an organic cause are indicated and may include curettage. This also applies in the case of spotting which occurs at regular intervals in several consecutive cycles or which occurs for the first time after long use of Co-cyprindiol Tablets.
Effect on blood chemistry:
The use of oral contraceptives may influence the results of certain laboratory tests including biochemical parameters of liver, thyroid, adrenal and renal function, plasma levels of carrier proteins and lipid/lipoprotein fractions, parameters of carbohydrate metabolism and parameters of coagulation and fibrinolysis. Laboratory staff should therefore be informed about oral contraceptive use when laboratory tests are requested.
Refer to Section 4.4 for further information.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme.
4.9 Overdose
Overdose may cause nausea, vomiting and, in females, withdrawal bleeding. There are no specific antidotes and further treatment should be symptomatic.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Antiandrogens and estrogens ATC code: G03HB01
Co-cyprindiol Tablets block androgen-receptors. They also reduce androgen synthesis both by a negative feedback effect on the hypothalamo-pituitary-ovarian systems and by the inhibition of androgen-synthesising enzymes.
Although Co-cyprindiol Tablets also act as an oral contraceptive, they are not recommended in women solely for contraception, but should be reserved for those women requiring treatment for the androgen-dependent skin conditions described.
5.2 Pharmacokinetic properties
Cyproterone acetate:
Following oral administration cyproterone acetate is completely absorbed over a wide dose range. The ingestion of 2mg cyproterone in combination with 0.035mg of ethinylestradiol gave a maximum serum level of 15ng cyproterone acetate/ml at 1.6 hours. Thereafter drug serum levels decrease in two disposition phases characterised by half-lives of 0.8 hours and 2.3 days. The total clearance of cyproterone acetate from serum was determined to be 3.6ml/min/kg. Cyproterone acetate is metabolised by various pathways including hydroxylations and conjugations. The main metabolite in human plasma is the 15-hydroxy derivative.
Some of the dose was excreted unchanged with the bile fluid. Most is excreted in the form of metabolites at a urinary to biliary ratio of 3:7. The renal and biliary excretion was determined to proceed with half-life of 1.9 days. Metabolites from plasma were eliminated at a similar rate (half-life of 1.7 days). Cyproterone acetate is almost exclusively bound to plasma albumin only about 3.5 - 4.0% of total drug levels are present unbound. Because protein binding is non-specific, changes in sex hormone binding globulin (SHBG) levels do not affect cyproterone acetate pharmacokinetics.
According to the long half-life of the terminal disposition phase from plasma (serum) and the daily intake, cyproterone acetate accumulates during one treatment cycle. Mean maximum drug serum levels increased from 15ng/ml (day 1) to 21ng/ml and 24ng/ml at the end of the treatment cycles 1 and 3 respectively. The area under the concentration versus time profile increased
2.2 fold (end of cycle 1) and 2.4 fold (end of cycle 3). Steady state conditions were reached after about 16 days. During long term treatment cyproterone acetate accumulates over treatment cycles by a factor of 2.
The absolute bioavailability of cyproterone acetate is almost complete (88% of dose). The relative bioavailability of cyproterone acetate (in combination with 0.035mg of ethinylestradiol) was 109% when compared to an aqueous microcrystalline suspension.
Ethinylestradiol:
Orally administered ethinylestradiol is rapidly and completely absorbed. Following ingestion of 0.035mg of ethinylestradiol in combination with 2mg of cyproterone, maximum drug serum levels of about 80pg/ml are reached at 1.7 hours. Thereafter ethinylestradiol plasma levels decrease in two phases characterised by half-lives of 1 - 2 hours and about 20 hours. For analytical reasons these parameters can only be calculated for higher dosages.
For ethinylestradiol an apparent volume of distribution of about 5 l/kg and a metabolic clearance rate from plasma of about 5 ml/min/kg were determined.
Ethinylestradiol is highly, but non-specifically bound to serum albumin, only 2% of the drug levels are present unbound. During absorption and first liver passage ethinylestradiol is metabolised resulting in a reduced absolute and variable oral bioavailability. Unchanged drug is not excreted. Ethinylestradiol metabolites are excreted at a urinary to biliary ratio of 4:6 with a half-life of about 1 day.
According to the half-life of the terminal disposition phase from plasma and the daily ingestion, steady state plasma levels are reached after 3 - 4 days and are higher by 30 - 40% as compared to a single dose. The relative bioavailability (reference: aqueous microcrystalline suspension) of ethinylestradiol was almost complete.
The systemic bioavailability of ethinylestradiol might be influenced in both directions by other drugs. There is, however, no interaction with high doses of vitamin C.
Ethinylestradiol induces the hepatic synthesis of SHBG and corticosteroid binding globulin (CBG) during continuous use. The extent of SHBG induction, however, is dependent upon the chemical structure and dose of the co-administered progestin. During treatment with this product, SHBG concentrations in serum increased from about 100nmol/l to 300nmol/l and the serum concentrations of CBG were increased from about 50pg/ml to 95p,g/ml.
5.3 Preclinical safety data
There are no preclinical safety data which could be of relevance to the prescriber and which are not already included in other relevant sections of the SPC.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Tablet core:
Lactose monohydrate Maize starch Povidone K25 Talcum
Magnesium stearate (E 572)
Tablet coat:
Sucrose
Calcium carbonate (E 170)
Macrogol 6000 Povidone K90
Titanium dioxide (E 171) Glycerol 85% (E 422) Montan glycol wax Iron oxide yellow (E 172)
6.2 Incompatibilities
Not applicable
6.3 Shelf life
3 years
6.4 Special precautions for storage
Store below 30°C. Store in the original package.
6.5 Nature and contents of container
PVC/Aluminium blister packs or PVC/PVDC/Aluminium blister-packs each containing 21 tablets. Each carton contains either 1 or 3 blister strips.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
None
7 MARKETING AUTHORISATION HOLDER
Teva UK Limited Brampton Road Hampden Park Eastbourne East Sussex BN22 9AG UK
8 MARKETING AUTHORISATION NUMBER(S)
PL 00289/1604
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
01/11/2007 / 26/09/2013
DATE OF REVISION OF THE TEXT
26/09/2013