Colifin 1 Miu Powder For Nebuliser Solution

What is in this leaflet:
1. What ColiFin® is and what it is used for
2. What you need to know before you use ColiFin®
3. How to use ColiFin®
4. Possible side effects
5. How to store ColiFin®
6. Contents of the pack and other information

The recommended dose is:
|
Proposed Dose |
Maximum dose per day | |
|
Adults, Adolescents (age 12 years to 17 years), Children (age 2 years to 11 years) |
1 - 2 million units two to three times per day |
6 million units |
|
Children less than 2 years |
0.5 - 1 million units twice daily |
2 million units |
Package leaflet: Information for the user
ColiFin® 1 Million International Units Powder for Nebuliser Solution
Colistimethate sodium
Read all of this leaflet carefully before you
start using this medicine because it contains
important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
DWHAT COLIFIN® IS AND WHAT IT IS USED FOR
ColiFin® is given as an inhalation to treat chronic chest infections in patients with cystic fibrosis. ColiFin® is used when these infections are caused by specific bacteria called Pseudomonas aeruginosa.
WHAT YOU NEED TO KNOW BEFORE YOU USE COLIFIN®
Do not use ColiFin®
• If you are allergic (hypersensitive) to colistimethate sodium, colistin or to other polymyxins.
Warnings and precautions
Talk to your doctor, pharmacist or nurse before using ColiFin®
• If you have or have had kidney problems
• If you suffer from myasthenia gravis
• If you suffer from porphyria
• If you suffer from asthma
In premature and new-born babies, special care should be taken when using ColiFin® as the kidneys are not yet fully developed.
Coughing and chest tightness may lead to discontinuation. This may be relieved by using an inhaled bronchodilator (e.g. salbutamol) before using ColiFin®. Your doctor will supervise your first dose of ColiFin® and check your lung function before and after dosing.
If chest tightness occurs despite the use of a bronchodilator, please tell your doctor because this may indicate an allergic reaction and the treatment should be discontinued.
During treatment with ColiFin® neurotoxicity may occur with the possibility of dizziness, confusion or visual disturbance. If you do experience any side effects such as dizziness, confusion or visual disturbance or also ones not listed in this patient information, please tell your doctor.
Other medicines and ColiFin®
• medicines which can affect how your kidneys function. Taking such medicines at the same time as ColiFin® can increase the risk of damage to the kidneys
• medicines which can affect your nervous system. Taking such medicines at the same time as ColiFin® can increase the risk of side effects in your nervous system
• medicines called muscle relaxants, often used during general anaesthesia. ColiFin® can increase the effects of these medicines. If you have a general anaesthetic, let your anaesthetist know that you are having ColiFin®
If you suffer from myasthenia gravis and are also taking other antibiotics called macrolides (such as azithromycin, clarithromycin or erythromycin) or antibiotics called fluoroquinolones (such as ofloxacin, norfloxacin and ciprofloxacin), taking ColiFin® further increases the risk of muscle weakness and breathing difficulties.
Having colistimethate sodium as an infusion at the same time as receiving ColiFin® as an inhalation can increase your risk of side effects.
ColiFin® should not be mixed with any other drugs in the nebuliser!
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
ColiFin® has moderate influence on the ability to drive and use machines. During treatment with ColiFin® neurotoxicity may occur with the possibility of dizziness, confusion or visual disturbance. If you do experience any side effects, such as dizziness, confusion or visual disturbance do not drive or operate machines and talk to your doctor or pharmacist.
HOW TO USE COLIFIN®
ColiFin® is for inhalation use.
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
Your doctor may decide to adjust the dose depending on your circumstances. If you also take other inhaled medicines, your doctor will tell you which order to take them in.
Please note ColiFin® is also available as 2 million units vial that might be a more suitable dose depending on the posology prescribed by the doctor.
For use in children younger than 2 years the PARI LC SPRINT® BABY (red nozzle insert) with mask is recommended.
Your doctor will tell you how long your treatment with ColiFin® will last. Do not stop treatment early because when treating bacterial infections it is important to complete the full course of treatment to reduce the risk of resistance formation of the infectious bacteria.
Preparation for inhalation treatment If you are treating yourself at home, your doctor or nurse will show you how to use ColiFin® in your nebuliser when you first start this treatment.
To start your treatment, you will need the following:
• One 10 ml vial of ColiFin® 1 million units
• Sterile sodium chloride 9 mg/ml (0.9%) solution 3 ml for dissolving the powder
• A nebuliser appropriate for inhalation use of ColiFin® (e.g. eFlow®rap/'d or PARI LC SPRINT®)
It is important that your nebuliser system functions properly before starting your treatment with ColiFin®.
Read carefully the instructions for use of the nebuliser for further information on handling the nebuliser system.
Place the components of your nebuliser on a clean flat surface and follow the manufacturer's instructions for use.
Preparing your ColiFin® for Inhalation
ColiFin® must be used immediately after dissolution. Do not dissolve ColiFin® until ready to administer a dose (see also section 5).
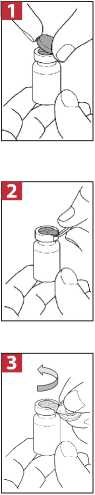
Step 1: Take one vial of ColiFin® and gently tap the glass vial so that the powder settles to the bottom. This helps ensure you get the proper dose of medication. Open the vial by lifting up the red plastic overcap on the top (Figure 1).
Step 2: Pull down the red plastic overcap together with the metal ring (Figure 2).
Step 3: Tear the red overcap with a twisting movement in the direction of the arrow. Correct performance will allow you to tear the metal ring open. Tear the metal ring sideways so that the breaking point on the opposite side bends. Remove the metal ring from the vial. Safely dispose of the ring and overcap.
5
|
1 million units/vial: |
White powder in a 10 ml colourless glass vial with a red cap. |
|
Also available: 2 million units/vial: |
White powder in a 10 ml colourless glass vial with a lavender cap. |
Step 4: Carefully remove the rubber stopper.
• Add 3 ml of sterile sodium chloride 9 mg/ml (0.9%) solution to ColiFin® 1 million units vial (red cap)
In order to avoid foaming, shake the vial gently until all powder is dissolved. Do not use ColiFin® if you notice visible particles in the solution after dissolution.
Using your ColiFin®
ColiFin® is for inhalation use with an appropriate nebuliser (e.g. eFlow®rap/d or PARI LC SPRINT®).
For more detailed information on correct use of the selected nebuliser follow the instruction manual of the nebuliser.
Inhalation should take place in a well ventilated room.
After inhalation of ColiFin®
Please refer to the manufacturer's instructions for use of the nebuliser for cleaning and disinfecting instructions.
If you use more ColiFin® than you should
If you have used more ColiFin® than you should, talk to a doctor or pharmacist immediately. If too much ColiFin® is accidentally given, the effects can be serious and can include kidney problems, muscle weakness and difficult (or even stopping) breathing.
If you forget to use ColiFin®
If you are treating yourself and have missed any doses, you should give the missed dose as soon as you remember and then give the next dose at 8 or 12 hours later and carry on from there as instructed.
Do not use a double dose to make up for a forgotten dose.
If you stop using ColiFin®
You should not stop using ColiFin® as prescribed without first consulting with your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4 POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
An allergic reaction is possible. Serious allergic reactions can happen even with the very first dose and can include rapid development of rashes, swelling of face, tongue and neck, inability to breathe due to narrowing of airways and loss of consciousness. Urgent medical attention is needed.
If you think you are having an allergic reaction to ColiFin®, tell your doctor immediately.
Some side effects can be serious.
Very common (may affect more than 1 in 10 people)
• A feeling of tightness in the chest due to narrowing of the airways (may not always be a true allergic reaction)
Not known (frequency cannot be estimated from the available data)
• Patients with severe renal impairment and higher dosages may experience side effects known for intravenous administration
• Confusion
• Psychotic disorder
• Visual disturbance
• Dizziness
If you experience any of these, tell your doctor straight away.
Other possible side effects:
Very common (may affect more than 1 in 10 people)
• mouth or throat sore
• Coughing
• Shortness of breath
• Wheezing
• Worsening of lung function test results
• Transient absence of spontaneous respiration
If any of these affects you severely, tell your doctor.
Not known (frequency cannot be estimated from the available data)
• Subjective skin sensations
• Speech disorder
• Vertigo
• Renal failure
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme. Website: www.mhra.gov.uk/yellowcard By reporting side effects you can help provide more information on the safety of this medicine.
HOW TO STORE COLIFIN®
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the vial label. The expiry date refers to the last day of that month.
Do not store above 25 °C.
Keep the vials in the outer carton in order to protect from light.
ColiFin® solution for nebulisation should be used immediately after preparation. If this is not possible, a ColiFin® solution should not be stored above 25 °C and not longer than 24 hours.
If not used immediately, in-use storage times and conditions are the responsibility of user.
Any remaining solution should be discarded.
For single use only.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
I CONTENTS OF THE PACK AND OTHER INFORMATION
What ColiFin® contains
• The active substance is colistimethate sodium.
• Each 10 ml vial contains 1 million units equivalent to 80 mg of colistimethate sodium.
What ColiFin® looks like and contents of the pack
ColiFin® is a powder for nebuliser solution.
The product is available in the following pack size:
Cardboard box containing 8 cardboard boxes of 7 vials each (56 vials) plus 2 cardboard boxes INQUA® NaCl 0.9% for inhalation containing 30 vials a 3 ml each (60 vials) plus eFlow®rap/d nebuliser handset
Marketing Authorisation Holder and Manufacturer
PARI Pharma GmbH Moosstr. 3 82319 Starnberg Germany
Tel.: +49(0)89 74 28 46-10 E-Mail: info@paripharma.com
For any information about this medicine, please contact the local representative of the Marketing Authorisation Holder:
United Kingdom:
PARI MEDICAL Ltd.
Tel.: +44 (0) - 1932 341122
Nederland:
Tel.: +49 (0)89 74 28 46-10
Deutschland:
Tel.: +49 (0)89 74 28 46-10
Italia:
Tel.: +49 (0)89 74 28 46-10 Osterreich:
Tel.: +49 (0)89 74 28 46-10 Espania:
Tel.: +49 (0)89 74 28 46-10
This leaflet was last revised in 03/2016
200D2000D