Colifin 2 Miu Powder For Nebuliser Solution
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
ColiFin 2 MIU Powder for Nebuliser Solution
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each 10 ml vial contains 2 MIU equivalent to 160 mg of Colistimethate sodium
3 PHARMACEUTICAL FORM
Powder for Nebuliser Solution.
White powder
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
ColiFin is indicated for the management in adult and paediatric of chronic pulmonary infections due to Pseudomonas aeruginosa in patients with cystic fibrosis (see section 5.1).
Consideration should be given to official guidance on the appropriate use of antibacterial agents.
4.2 Posology and method of administration
It is recommended that colistimethate sodium (CMS) should be administered under the supervision of physicians with appropriate experience in its use.
Posology
The dosage can be adjusted depending on the severity of the condition and clinical response.
Recommended dose range:
Administration via inhalation
Adults, adolescents and children > 2 years
1-2 MIU two to three times per day (max. 6 MlU/day)
Children < 2 years
0.5-1 MIU twice daily (max. 2 MIU/day)
Relevant clinical guidance on treatment regimens, including duration of treatment, periodicity and co-administration of other antibacterial agents should be adhered to.
Older people
Dose adjustment is not considered necessary.
Renal impairment
Dose adjustment is not considered necessary, however, caution is advised in patients with renal impairment (see sections 4.4 and 5.2).
Hepatic impairment
Dose adjustment is not considered necessary.
Method of administration
For inhalation use.
For use in children younger than 2 years the PARI LC SPRINT Baby (red nozzle insert) with mask is recommended.
The content of a vial of ColiFin 2 MIU should be dissolved in 4 ml of sterile sodium chloride 9 mg/ml (0.9%) solution.
For instructions on dilution of the product before administration, see section 6.6.
Predicted drug delivery characteristics as studied in vitro with different nebuliser devices for ColiFin 2 MIU dissolved in 4 ml of sterile sodium chloride 9 mg/ml (0.9%) solution (min - max).
Table 1
|
Nebuliser system |
PARI LC SPRINT with PARI BOY S compressor |
eFlowrapid nebuliser |
|
Total Drug Delivered |
65 mg CMS (59.9 - 72.5) |
58 mg CMS (54.6 - 62.5) |
|
Fine Particle Mass < 5 pm |
39.1 mg CMS (36.0 - 45.8) |
40 mg CMS (36.8 - 43.2) |
|
Drug Delivery Rate |
6.7 mg CMS/min (5.7 - 8.6) |
9.5 mg CMS/min (8.1 - 10.7) |
|
Mass Median Aerodynamic Diameter |
4.1 pm (3.9 - 4.4) |
4.0 pm (3.8 - 4.3) |
|
Geometric Standard Deviation |
2.1 |
1.6 |
• The nebulisation time may increase during 60 cycles of nebulisation from ~ 3 minutes to ~ 4.5 minutes with eFlowrapid nebuliser handset.
• The nebuliser must be kept horizontally during operation.
• The patient should sit in an upright position during inhalation. Inhalation should be performed applying a normal breathing pattern without interruption.
• The nebuliser must be cleaned and disinfected after use as described in the instructions of use of the corresponding nebuliser.
There is no information available in respect of pulmonary inhalation and deposition patterns across nebuliser systems that have not been studied in the development programme; the use of an alternative untested nebuliser system may alter the pulmonary deposition of the active substance, this in turn may alter the efficacy and safety of the medicinal product.
Colistimethate sodium undergoes hydrolysis to the active substance colistin in aqueous solution.
For special precautions for disposal and handling of reconstituted solutions, see section 6.6.
If other treatments are being taken, they should be taken in the order recommended by the physician.
Dose conversion table:
In the EU, the dose of colistimethate sodium (CMS) must be prescribed and administered only as International Units (IU). The product label states the number of IU per vial.
Confusion and medication errors have occurred because of the different expressions of dose in terms of potency. The dose is expressed in the US, and other parts of the world, as milligrams of colistin base activity (mg CBA).
The following conversion table is prepared for information and the values must be considered nominal and approximate only.
CMS conversion Table 2
|
Potency |
~ mass of CMS (mg)* | |
|
IU |
~ mg CBA | |
|
12,500 |
0.4 |
1 |
|
150,000 |
5 |
12 |
|
1,000,000 |
34 |
80 |
|
2,000,000 |
68 |
160 |
|
4,500,000 |
150 |
360 |
|
9,000,000 |
300 |
720 |
*Nominal potency of the drug substance
12,500 IU/mg
4.3 Contraindications
Hypersensitivity to the active substance, colistin or other polymyxins.
4.4 Special warnings and precautions for use
Coughing and bronchospasm
Coughing and bronchospasm may occur on inhalation of antibiotics.
It is recommended to administer the first dose under medical supervision. Pre-dosing with a bronchodilator is recommended and should be routine, especially if this is part of the patient's current therapeutic regimen. FEV1 should be evaluated pre and post dosing. If there is evidence of colistimethate sodium induced bronchial hyper reactivity in a patient not receiving pre-treatment bronchodilators, the test should be repeated on a separate occasion using a bronchodilator. Evidence of bronchial hyper reactivity in the presence of a bronchodilator may indicate an allergic response and ColiFin should be discontinued. Bronchospasm should be treated as medically indicated.
Bronchial hyper reactivity in response to colistimethate sodium may develop with continued use over time and it is recommended that pre- and post-treatment FEV1 are evaluated at regular clinic visits.
In case of hypersensibility with respect to the recommended doses and volumes more diluted solutions should be used by adding about 1 - 3 ml isotonic saline to the recommended volumes and dose strengths.
Nephrotoxicity/ neurotoxicity
Nephrotoxicity or neurotoxicity may occur if the recommended parenteral dose is exceeded. The risk is reduced due to the low bioavailability during inhalation, but ColiFin should be used with caution in patients with renal impairment. Appearance of neurotoxic reactions as well as the renal function should be monitored.
In premature and newborn infants special care should be employed as renal function is only insufficiently developed in this population.
Renal impairment
Colistimethate sodium is renally excreted and is nephrotoxic if high serum concentrations are achieved. Whilst this is unlikely during inhalation therapy, serum concentration estimations are recommended especially in patients with renal impairment.
Microbial Resistance
Colistimethate sodium acquired resistance in mucoid Pseudomonas aeruginosa during clinical use has been reported. Susceptibility testing should be performed on patients who are treated on a long term basis, at regular clinic visits, and whenever a patient experiences an exacerbation (see Section 5.1).
Other
Colistimethate sodium should be used with extreme caution in patients with myasthenia gravis because of potential for drug induced neuromuscular blockade (see section 4.5).
Colistimethate sodium should be used with extreme caution in patients with porphyria.
4.5 Interaction with other medicinal products and other forms of interaction
Concomitant use of colistimethate sodium with other medicinal products of neurotoxic and/or nephrotoxic potential (e.g. cephalosporins, aminoglycosides, ciclosporin) including those which are administered by the IV or IM routes should be avoided.
During concomitant use of inhalation narcotics (e.g. ether, halothan), muscle relaxants and aminoglycosids with colistimethate sodium appearance of neurotoxic reactions should be thoroughly monitored due to prolongation effect of the inhalation of narcotics.
Due to the effects of colistimethate sodium on the release of acetylcholine, nondepolarising muscle relaxants should be used with extreme caution in patients receiving colistimethate sodium as their effects could be prolonged.
Co-treatment with colistimethate sodium and macrolides such as azithromycin, clarithromycin and erythromycin, or fluoroquinolones such as norfloxacin, ciprofloxacin and ofloxacin should be undertaken with caution in patients with myasthenia gravis (see section 4.4).
Caution should be taken with concomitant use with other formulations of colistimethate sodium as there is a possibility of summative toxicity.
4.6 Fertility, pregnancy and lactation
There are no adequate data from the use of colistimethate sodium in pregnant women. Single dose studies in human pregnancy showed that colistimethate sodium crosses the placental barrier and there may be a risk of fetal toxicity if repeated doses are given to pregnant patients. Animal studies are insufficient with respect to the effect of colistimethate sodium on reproduction and development (see section 5.3). Colistimethate sodium should not be used in pregnancy unless the benefit to the mother outweighs the potential risk to the fetus.
Colistimethate sodium is secreted in breast milk. Colistimethate sodium should be administered to breastfeeding women only when clearly indicated and the benefit to the mother outweighs the potential risk to the child.
4.7 Effects on ability to drive and use machines
ColiFin has moderate influence on the ability to drive and use machines. During treatment with colistimethate sodium neurotoxicity may occur with the possibility of dizziness, confusion or visual disturbance. Patients should be warned not to drive or operate machinery if these effects occur.
4.8 Undesirable effects
The most common undesirable effects following nebulisation of colistimethate sodium are coughing and bronchospasm in approximately 10% of patients. In cystic fibrosis patients treated by IV or IM injection neurological events have been reported in up to 27% of patients.
Tabulated list of adverse reactions
Adverse drug reactions in Table 3 are listed according to system organ classes in MedDRA. Within each system organ class, the adverse drug reactions are ranked by frequency, with the most frequent reactions first. Within each frequency grouping, adverse reactions are presented in order of decreasing seriousness. In addition, the corresponding frequency category using the following convention (CIOMS III):
Very common (>1/10); Common (>1/100 to <1/10); Uncommon (>1/1,000 to <1/100); Rare (>1/10,000 to <1/1,000); Very rare (<1/10,000); not known (cannot be estimated from the available data).
The likelihood of adverse events may be related to the age, renal function and condition of the patient.
Table 3 Adverse reactions
|
System Organ Class |
Frequency category |
Adverse Reactions |
|
Psychiatric disorders |
Not known |
Confusional state Psychotic disorder |
|
Nervous system disorders |
Not known |
Dizziness Paraesthesia Dysarthria Autonomic nervous system imbalance |
|
Eye disorders |
Not known |
Visual disturbance |
|
Ear and labyrinth disorders |
Not known |
Vertigo |
|
Respiratory, thoracic and mediastinal disorders |
Very common |
Pharyngolaryngeal pain Pharyngolaryngeal discomfort |
|
Cough Dyspnoea Wheezing Shortness of breath Forced expiratory volume decreased Apnoea | ||
|
Renal and urinary disorders |
Not known |
Renal failure |
Patients with severe renal impairment and higher dosages may experience side effects known for intravenous administration.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme.
Website: www.mhra.gov.uk/yellowcard.
4.9 Overdose
Symptoms
Overdose may cause muscular weakness, apnoea and possible respiratory arrest as well as acute renal failure characterised by decreased urine output and increased serum concentrations of BUN and creatinine.
There is no specific antidote.
Treatment
Management of overdose is by means of supportive treatment and measures to increase the rate of elimination of colistin such as mannitol diuresis, prolonged haemodialysis or peritoneal dialysis.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Other antibacterials, polymyxins, ATC code: J01X B01 Mechanism of action
Colistimethate sodium is a cyclic polypeptide antibiotic derived from Bacillus polymyxa var. colistinus. The polymyxin antibiotics are cationic agents that work by damaging the cell membrane. The resulting physiological effects are lethal to the bacterium. Polymyxins are selective for Gram-negative bacteria that have a hydrophobic outer membrane.
Breakpoints
Susceptible (S) < 4 mg/l Resistant (R) : 8 mg/l
Resistance
Resistant bacteria are characterised by modification of the phosphate groups of lipopolysaccharide that become substituted with ethanolamine or aminoarabinose. Naturally resistant Gram-negative bacteria, such as Proteus mirabilis and Burkholderia cepacia, show complete substitution of their lipid phosphate by ethanolamine or aminoarabinose.
Colistimethate sodium acquired resistance in mucoid Pseudomonas aeruginosa has been reported to be approximately 3%. Susceptibility testing should be performed on patients who are treated on a long term basis.
Cross resistance
Cross resistance between colistimethate sodium and polymyxin B is expected. Since the mechanism of action of the polymyxins is different from that of other antibacterial agents, resistance to colistimethate sodium and polymixin by the above mechanism alone would not be expected to result in resistance to other drug classes.
Susceptibility
The prevalence of acquired resistance may vary geographically and with time for selected species and local information on resistance is desirable, particularly when treating severe infections. As necessary, expert advice should be sought when the local prevalence of resistance is such that the utility of the agent in at least some types of infections is questionable.
Table 4
Commonly susceptible species
Pseudomonas aeruginosa
Species for which acquired resistance may be a problem
Enterobacter species Klebsiella species
Inherently resistant organisms
Brucella species
Burkholderia cepacia and related species.
Neisseria species Proteus species Providencia species Serratia species
Anaerobes
All Gram positive organisms
Aerosol characteristics
PARI LC PLUS and PARI LC STAR are nebulisers which had been used in the past for nebulisation of colistimethate sodium. These jet nebulisers were compared with the eFlowrapid for the nebulisation of 2 MIU colistimethate sodium dissolved in 4 ml of sterile sodium chloride 9 mg/ml (0.9%) solution by in-vitro testing:
Table 5
|
Nebuliser |
eFlowrapid |
PARI LC PLUS |
PARI LC STAR |
|
Total Drug Delivered |mg ± 95% CI*1 |
57.8 ± 1.56 |
61.3 ± 1.25 |
60.1 ± 1.20 |
|
Drug Delivery Rate [mg/min ± 95% CI*] |
9.5 ± 0.51 |
6.6 ± 0.41 |
4.1 ± 0.15 |
*Confidence interval
5.2 Pharmacokinetic properties
Absorption
Absorption from the gastrointestinal tract is neglible.
When given by nebulisation, variable absorption has been reported that may depend on the aerosol particle size, nebuliser system and lung status. Studies in healthy volunteers and patients with various infections have reported serum levels from nil to potentially therapeutic concentrations of 4mg/l or more. Therefore, the possibility of systemic absorption should always be borne in mind when treating patients by inhalation.
Distribution
After the administration to patients with cystic fibrosis of 7.5 mg/kg/day in divided doses given as 30-min i.v. infusions to steady state the Cmax was determined to be 23 ± 6 mg/l and Cmin at 8 h was 4.5 ± 4 mg/l. In another study in similar patients given 2 MIU every 8 hours for 12 days the Cmax was 12.9 mg/l (5.7 - 29.6 mg/l) and the Cmin was 2.76 mg/l (1.0 - 6.2 mg/l). In healthy volunteers given a bolus injection of 150 mg (2 MIU approx.) peak serum levels of 18 mg/l were observed 10 minutes after injection.
Protein binding is low. Polymyxins persist in the liver, kidney, brain, heart and muscle. One study in cystic fibrosis patients gives the steady-state volume of distribution as 0.09 l/kg.
Biotransformation
Colistimethate sodium is converted to the base in vivo. As 80% of the dose can be recovered unchanged in the urine, and there is no biliary excretion, it can be assumed that the remaining active substance is active in the tissues. The mechanism is unknown.
Elimination
The main route of elimination after parenteral administration is by renal excretion with 40% of a parenteral dose recovered in the urine within 8 hours and around 80% in 24 hours. Because Colistimethate sodium is largely excreted in the urine, dose reduction is required in renal impairment to prevent accumulation. Refer to the table in Section 4.2.
After intravenous administration to healthy adults the elimination half-life is around 1.5 hrs. In a study in cystic fibrosis patients given a single 30-minute intravenous infusion the elimination half-life was 3.4 ± 1.4 hrs.
The elimination of colistimethate sodium following nebulisation has not been studied. A study in cystic fibrosis patients failed to detect any colistimethate sodium in the urine after 1 MIU were inhaled twice daily for 3 months.
Colistimethate sodium kinetics appear to be similar in children and adults, including the elderly, provided renal function is normal. Limited data are available on use in neonates which suggest kinetics are similar to children and adults but the possibility of higher peak serum levels and prolonged half-life in these patients should be considered and serum levels monitored.
Table 6 Serum concentrations and pharmacokinetics in 5 patients receiving inhaled colistimethate sodium
|
Parameter |
160 mg (approximately 2 MIU) nebulised CMS |
|
AUC0-4 (h/mg/L) |
165.9 ± 76.5 |
|
Cmax (mg/L) |
0.051 ± 0.0244 |
|
Tmax (h) |
1.9 ± 1.2 |
|
Ka (h-1 ) |
3.0 ± 1.8 |
|
t* (h) |
10.4 ± 3.6 |
|
Cl/F |
0.27 ±0.15 |
5.3 Preclinical safety data
Data on potential genotoxicity are limited and carcinogenicity data for colistimethate sodium are lacking. Colistimethate sodium has been shown to induce chromosomal aberrations in human lymphocytes, in vitro. This effect may be related to a reduction in mitotic index, which was also observed.
Reproductive toxicity studies in rats and mice do not indicate teratogenic properties. However, Colistimethate sodium given intramuscularly during organogenesis to rabbits at 4.15 and 9.3 mg/kg resulted in talipes varus in 2.6% and 2.9% of fetuses respectively. These doses are 0.5 and 1.2 times the maximum daily human dose. In addition, increased resorption occured at 9.3 mg/kg.
There are no other preclinical safety data of relevance to the prescriber which are additional to safety data derived from patient exposure and already included in other sections of the SmPC
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
None.
6.2 Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
6.3 Shelf life
3 years
Reconstituted solutions:
Hydrolysis of colistimethate is significantly increased when reconstituted and diluted below its critical micelle concentration of about 80,000 IU per ml.
Solutions below this concentration should be used immediately.
ColiFin can be stored after reconstitution with sterile sodium chloride 9 mg/ml (0.9%) solution for 24 hours below 25°C.
From a microbiological point of view, unless the method of opening/ reconstitution/ dilution precludes the risk of microbial contamination, the product should be used immediately.
If not used immediately, in-use storage times and conditions are the responsibility of user.
Please follow the manufacturer’s instructions on correct use of a nebuliser selected to be used with ColiFin solution.
6.4 Special precautions for storage
Do not store above 25°C. Keep the vials in the outer carton in order to protect from light.
For storage conditions of the reconstituted medicinal product, see section 6.3.
6.5 Nature and contents of container
ColiFin 2 MIU: 10 ml colourless glass vials with lavender flip-tear-off caps.
Pack size supplied:
• Cardboard box containing 8 cardboard boxes of 7 vials each (56 vials)
plus 2 cardboard boxes INQUA NaCl 0.9% for inhalation containing 30 vials at 4 ml each (60 vials)
plus eFlowrapid nebuliser handset
6.6 Special precautions for disposal
The required dose of ColiFin should be dissolved in the respective volume of sterile sodium chloride 9 mg/ml (0.9%) solution. During reconstitution the solution should be swirled gently to avoid frothing. The resulting solution for nebulisation should be carefully transferred into the medication reservoir of the nebuliser. For further instructions for handling and use consult the instructions for use of the nebuliser.
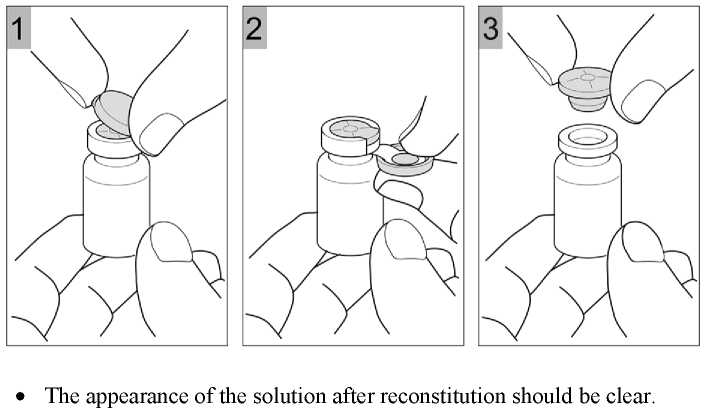
• Nebulisation should take place in a well ventilated room.
• The solution is for single use only and any remaining solution should be discarded.
• For more detailed information on the device refer to the instruction manual of the nebuliser.
• For instructions on dilution of the product before administration, see section 4.2.
7 MARKETING AUTHORISATION HOLDER
PARI Pharma GmbH, Moosstr. 3
82319 Starnberg Germany
Tel.:
Fax:
E-Mail:
+49 (0) 81 51 / 279-279 +49 (0) 81 51 / 279-101 info@pari.de
8
9
10
MARKETING AUTHORISATION NUMBER(S)
PL 32288/0002
DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
26/11/2014
DATE OF REVISION OF THE TEXT
26/11/2014