Combiprasal 0.5 Mg / 2.5 Mg Nebuliser Solution
PACKAGE LEAFLET
TRADENAME 0.5 mg / 2.5 mg nebuliser solution Ipratropium bromide and salbutamol
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What TRADENAME is and what it is used for
2. What you need to know before you use TRADENAME
3. How to use TRADENAME
4. Possible side effects
5. How to store TRADENAME
6. Contents of the pack and other information
1. What TRADENAME is and what it is used for
The name of your medicine is TRADENAME. You use it with a device called a ‘nebuliser’. This changes your medicine into a mist for you to breathe in.
The active substances are ipratropium bromide and salbutamol. Both belong to a group of medicines called bronchodilators. They work by opening up your airways and therefore making breathing easier.
TRADENAME is used in adults and children over 12 years to treat long term breathing problems (e.g. chronic bronchitis and emphysema).
TRADENAME alleviates wheezing when breathing, shortness of breath and tightness in the chest.
2. What you need to know before you use TRADENAME Do not use TRADENAME
- if you are allergic to salbutamol, ipratropium bromide or atropine (including medicines similar to atropine) or any of the other ingredients of this medicine (listed in section 6).
- if you have heart rhythm disorder including a very fast heart beat.
- if you know that your heart is enlarged or if you have a heart problem called ‘hypertrophic obstructive cardiomyopathy’. This is where the wall between the two sides of the heart gets bigger and blocks the blood flow.
Warnings and precautions
Talk to your doctor or pharmacist before using TRADENAME
- if you have glaucoma (an eye disease with elevated intraocular pressure) or have been told that you may develop it or suffer another eye disease. Your doctor may advise you to protect your eyes when you use TRADENAME.
- if you are a man who has prostate problems or has problems passing water.
- if you have heart disease or have had a recent heart attack, have arterial problems or get pain in the legs when walking.
- if you have diabetes.
- if you have an over-active thyroid gland.
- if you have cystic fibrosis.
- if you have a tumour of adrenal gland.
Children
TRADENAME is not recommended in children below 12 years of age as safety and efficacy have not been established in this age group.
Particularly in children it is recommended to pay attention to proper oral hygiene and perform regular dental checkups to avoid caries.
Impact in case of misuse for doping purposes:
The use of TRADENAME may lead to positive results in doping tests.
The use of TRADENAME as a doping agent may be hazardous to health.
If any of the conditions mentioned above apply to you, talk to your doctor before using TRADENAME. Other medicines and TRADENAME
Some other medicines can interact with TRADENAME and enforce side effects or reduce the effect of TRADENAME.
In particular, tell your doctor or pharmacist if you are taking any of the following medicines:
- Medicines for asthma, including inhalers and tablets for asthma, medicines for treatment of an acute asthma attack, such as salbutamol and long-acting medicines for permanent treatment such as beclomethasone propionate, that may increase the effect of TRADENAME and the severity of side effects.
- Beta blockers, i.e. medicines that are commonly used to treat heart problems such as chest pain occurring on exertion (angina pectoris), irregular heartbeat or heart rhythm disorders and high blood pressure (hypertension). These include medicines such as propranolol.
- Medicines called ‘anti-cholinergics’. These can be used to treat colic pain, Parkinson’s disease, problems passing water or lack of control of your bladder or bowels.
- Certain medicines used for treatment of depression (these ‘antidepressants’ are given to patients who suffer from depression and anxiety). This class of medicines includes for example monoamine oxidase inhibitors (MAOIs, e.g. phenelzine) or tricyclic antidepressants (e.g. amitriptyline), that may increase the effect of salbutamol.
- Digoxin (to treat heart problems), which can result in heart rhythm disorders when used concomitantly with TRADENAME.
- Diuretics (so called ‘water tablets’).
- Corticosteroid tablets (medicines used in treatment of inflammatory diseases in the body, such as prednisolone), which may enhance the blockade of the respiratory tract.
- Anaesthetic agents may increase the sensitivity to adverse effects on the heart by salbutamol. If you are going to have surgery, you will be monitored carefully or your doctor may decide to stop the use of TRADENAME.
Tell your doctor or pharmacist if you are taking/using or have recently taken/used or might take/use any other medicines, including medicines obtained without a prescription.
If you are about to have surgery in the hospital, make sure you mention to the anaesthetist which medicines you are taking.
TRADENAME with food and drink
Food and drink do not have influence on the effect of TRADENAME.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Do not use TRADENAME if you are pregnant or breast-feeding, unless you and your doctor decide that the benefit to you outweighs any risk to your child.
Driving and using machines
You may feel dizzy or have difficulty in focusing or blurred vision whilst taking TRADENAME. If this happens do not drive or use tools or machines.
3. How to use TRADENAME
TRADENAME single-dose containers are intended for inhalation. The solution is nebulised and then inhaled through the mouth.
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
The recommended dose for adults and children over 12 years is:
Inhalation of the content of 1 single-dose unit (corresponding to 2.5 ml), three or four times a day.
Elderly patients should use the usual dose recommended for adults.
Use in children:
TRADENAME is not recommended for children under 12 years.
Your doctor or pharmacist will write on the box, how much of and how often you should use TRADENAME.
Do not use more than your doctor has told you. Tell your doctor if your breathing difficulties increase or if the medicine no longer provides you as much relief in breathing as before or if you use your emergency medicine more often than usual.
TRADENAME has been tested using the eFlow rapid electronic nebuliser and the PARI LC Sprint nebuliser. There is no information available on the use with other nebuliser systems. If a different nebuliser system is used, your doctor may need to adjust your dose.
How to use your nebuliser:
- Prepare your nebuliser by following the manufacturer’s instructions and the training by your doctor.
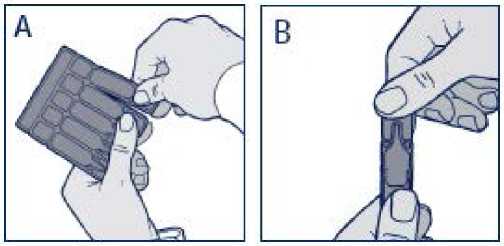
- Separate a new single-dose container from the strip by twisting and pulling carefully (Figure A). Never use a single-dose container if it is already open or if the liquid inside is discoloured.
- Put the remaining single-dose containers back in the sachet and close the sachet by crimping over the lap. Store the sachet in the carton.
- Hold the single-dose container upright and open by simply twisting off the top (Figure B).
Use with eFlow rapid electronic nebuliser:
- Unless otherwise instructed by your doctor, squeeze the content of one single-dose container into the nebuliser chamber.
- Close the nebuliser chamber by placing the cap on the nebuliser chamber so that the slots in the side of the cap are positioned above the notches in the chamber.
Press gently and turn the cap clockwise as far as it will go. The closing mechanism is functioning correctly if the cap seal rises with the turning motion to form a seal. Check that all parts are connected tightly and that the nebuliser chamber is sealed.
- Hold the nebuliser handset in your hand. Sit in an upright position and relax. Hold the mouthpiece between your teeth and close your lips around it. The lips should not touch the blue expiratory valve. Press the ON/OFF button on the control unit. A green LED beside the ON/OFF button lights up and an audible signal (1 beep) is emitted to indicate proper functioning.
Inhale and exhale through the mouthpiece as slowly and deeply as possible.
- The nebuliser device switches off automatically when the nebuliser solution is used up. When inhalation has been completed successfully, a tick will appear on the display.
Discard any remaining solution in the nebuliser chamber (about 1 ml cannot be nebulised and remains in the nebuliser chamber).
- When the inhalation session has ended, disconnect the power adapter plug from the socket.
Use with PARILC Sprint nebuliser:
- The nebuliser is operated with the PARI Boy SX compressor. Release the closure on the nebuliser upper section by pressing the thumb against the underside of the cap.
- Unless otherwise instructed by your doctor, squeeze the content of one single-dose container into the nebuliser chamber.
- Close the cap of the nebuliser. Make sure that the cap snaps into place. Ensure that all parts of the nebuliser are firmly connected to each other.
- Sit in an upright position and relax.
Switch the compressor on. Take the mouthpiece between your teeth and close your lips around it.
Breathe in through the mouthpiece as slowly and deeply as possible and then out again in your own time.
- Continue the inhalation until the solution in the nebuliser chamber is used up (signalled by a change in the sound of the nebuliser).
- Switch the compressor off as soon as you have finished the inhalation. Discard any remaining solution in the nebuliser chamber (some solution will remain after inhalation).
For eFlow rapid electronic nebuliser and PARI LC Sprint nebuliser:
Follow the manufacturer’s instructions for cleaning your nebuliser. It is important to keep your nebuliser clean.
Please read the full instructions for use of the nebuliser in the leaflet provided with the nebuliser system before starting the inhalation.
The solution must not be diluted or mixed with other medicines unless your doctor has advised you to.
Since the single-dose containers TRADENAME do not contain preservatives, it is important that the contents are used immediately after opening. Every time you use your nebuliser, a new single-dose container has to be opened.
Partly used, opened or damaged single-dose containers have to be discarded. Never use a single-dose container, which was already opened before.
It is important that you follow these instructions to avoid contamination of the inhalation solution in the single-dose containers.
Do not swallow or give this medicine by injection.
The solution or the mist must not reach your eyes.
Please talk to your doctor or your pharmacist if you feel that the effect of TRADENAME is too strong or too weak.
If you use more TRADENAME than you should
If you have used a slightly higher dose than usual you may experience heart palpitations and tremor. Other symptoms may include chest pain, variation of blood pressure, flushing, restlessness or dizziness. These effects usually disappear after a few hours. Because of a potential drop in blood potassium levels, your doctor may take a blood test from time to time. Consult your doctor if any of these symptoms worry you or persist.
If you use more of this medicine than you should, talk to a doctor or go to a hospital straight away. If you need to consult a doctor or a hospital, you should take all your medicines with you, including nonprescription medicines, which should be in their original packaging if possible. Take this leaflet with you and show it to the doctor.
If you forget to use TRADENAME
If you forget a dose, take it as soon as you remember it.
Do not take a double dose to make up for a forgotten dose.
If you stop using TRADENAME
Please do not stop using TRADENAME without first talking to your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some side effects may be serious and need urgent medical treatment.
Serious side effects:
If after using TRADENAME your wheezing worsens immediately or you have more difficulties in breathing or shortness of breath, stop using TRADENAME, and use your emergency medicine straight away. Do not take TRADENAME anymore and consult your doctor immediately. Your doctor will prescribe you another treatment for your condition. This side effect can occur rarely (may affect up to 1 in 1,000 people).
If you experience eye pain, stinging or discomfort to the eyes, blurred vision or reddened eyes, or if you notice halos or coloured spots, contact your doctor immediately as these symptoms, which can occur rarely (may affect up to 1 in 1,000 people), may require treatment.
Allergic reactions can occur rarely (may affect up to 1 in 1,000 people). If you think you might be allergic to TRADENAME or you believe that an allergic reaction on the inhalation solution has occurred, stop using TRADENAME immediately and see your doctor straight away.
Due to the active substance salbutamol contained in TRADENAME, a drop in the level of potassium in the blood (hypokalaemia) may occur rarely (may affect up to 1 in 1,000 people), which can cause muscle weakness, muscle twitching or heart rhythm disorders. This occurs more often when you use TRADENAME together with medicines to treat asthma, with corticosteroids for inhalation or in tablet form or with diuretics (‘water tablets’). Your doctor may check your blood potassium level from time to time.
Frequencies of other possible side effects:
Uncommon (may affect up to 1 in 100 people)
• Feeling nervous, shaky or dizzy
• Dry mouth
• Cough
• Headache
• Feeling sick (nausea)
• Throat irritation
• Increase in blood pressure
• Increased heart rate or uneven heart beats (palpitations)
• Voice problems (‘dysphonia’)
• Skin reactions
Rare (may affect up to 1 in 1,000 people)
• Irregular heart beat
• Regular but abnormally fast heart rate (supraventricular tachycardia)
• Chest pain (due to heart problems such as angina). Tell your doctor or pharmacist if this occurs but do not stop taking this medicine unless told to do so.
• Dilated pupils, increased pressure in the eye (glaucoma), swelling of the eyes, difficulty in focusing
• Increased sweating, rash, itching and urticaria
• Shortness of breath
• Dry throat, swelling of the throat, lips, face and tongue
• Difficulty in breathing or speaking due to a brief spasm of your vocal muscles
• Diarrhoea, constipation, being sick (vomiting) or other problems with your digestive system
• Inflammation or swelling of the mouth
• Muscle cramps, muscle weakness and pain
• Difficulty in passing water (urine)
• Feeling weak
• Fall in blood pressure
• Mental disorders, restlessness, hyperactivity in children, anxiety and mood changes.
Not known (frequency cannot be estimated from the available data)
• Dental caries
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store TRADENAME
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton, sachet and the vial after EXP. The expiry date refers to the last day of that month.
Do not store above 25°C. Do not freeze. Store in the original package in order to protect from light and evaporation.
Do not use this medicine if you notice that the liquid is discoloured.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information What TRADENAME contains
The active substances are:
Each single-dose container with 2.5 ml solution contains
- 0.5 mg ipratropium bromide (as monohydrate) and
- 2.5 mg salbutamol (as sulphate).
The other ingredients are:
Sodium chloride, sulphuric acid 10% (for pH-adjustment) and water for injections.
What TRADENAME looks like and contents of the pack
TRADENAME is a colourless solution that is filled in single-dose containers.
Strips of 5 single-dose containers are packed into a sachet. TRADENAME is available in pack sizes of 10, 20, 50, 60, 100 or 150 single-dose containers.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
<[To be completed nationally]>
This medicinal product is authorised in the Member States of the EEA under the following names:
Ireland: COMBIPRASAL 0.5 mg / 2.5 mg per 2.5 ml nebuliser solution
Germany: COMBIPRASAL 0,5 mg / 2,5 mg Losung fur einen Vernebler
The Netherlands: IPRASA 0,5 mg / 2,5 mg per 2,5 ml, verneveloplossing United Kingdom: COMBIPRASAL 0.5 mg / 2.5 mg nebuliser solution
This leaflet was last revised in May 2015. <[To be completed nationally]>
8