Combivent Udvs Nebuliser Solution
Out of date information, search another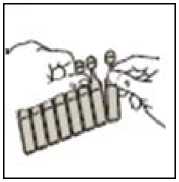
Patient Information Leaflet
Combivent® UDVs Nebuliser Solution
(ipratropium bromide and salbutamol sulphate)
The name of your medicine is Combivent UDVs Nebuliser Solution, but will be referred to as Combivent UDVs throughout this leaflet.
Read all of this leaflet carefully before you start taking this medicine
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or practice nurse.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects gets troublesome or serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Combivent UDVs is and what it is used for
2. Before you use Combivent UDVs
3. How to use Combivent UDVs
4. Possible side effects
5. How to store Combivent UDVs
6. Further information
1. What Combivent UDVs is and what it is used for
The name of your medicine is Combivent UDVs. You use it with a device called a ‘nebuliser'. This changes your medicine into a mist for you to breathe in. Combivent UDVs contains two different medicines called:
• Ipratropium bromide and
• Salbutamol sulfate
Both belong to a group of medicines called bronchodilators. They are used to make breathing easier in an illness called ‘chronic obstructive pulmonary disease' or COPD. They work by opening up your airways.
2. Before you use Combivent UDVs Do not use Combivent UDVs if:
• You are allergic (hypersensitive) to ipratropium or salbutamol or any of the other ingredients in Combivent UDVs. (Listed in section 6: Further information)
• You are allergic to similar medicines which contain atropine or medicines like atropine
• You have a heart problem called ‘hypertrophic obstructive cardiomyopathy'.
This is where the wall between the two sides of the heart gets bigger and blocks the blood flow
• You have a very fast heart beat (called ‘tachyarrythmia')
• You are pregnant, likely to get pregnant or are breast-feeding
Do not use if any of the above apply to you. If you are not sure, talk to your doctor or pharmacist before using Combivent UDVs.
Take special care with Combivent UDVs
Check with your doctor or pharmacist before using this medicine if:
• You have glaucoma, or have been told that you may develop it
• You have heart or circulation problems, or have had a recent heart attack
• You have diabetes
• You have an over-active thyroid gland
• You have problems passing water (urine)
• You are a man who has prostate problems
• You have cystic fibrosis
• You have ever had something called ‘pheochromocytoma'. This is a rare tumour which is not malignant. Using your inhaler can make the symptoms of this worse.
If you are not sure if any of these apply to you, talk to your doctor or pharmacist before using Combivent UDVs.
Taking other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription. This includes herbal medicines. This is because Combivent UDVs can affect the way some other medicines work.
Also some other medicines can affect the way Combivent UDVs works.
In particular, tell your doctor or pharmacist if you are taking any of the following medicines:
• Steroid medicines such as prednisolone
• Water tablets (also called ‘diuretics')
• Medicines for depression
• Medicines to help your breathing
• Medicines called ‘anti-cholinergics'. These can be used to treat colic pain, Parkinson's Disease, problems passing water or lack of control of your bladder or bowels
• Medicines called ‘beta blockers' such as propranolol. These can be used to treat heart problems, high blood pressure, anxiety or migraine
• Medicines called ‘beta mimetics' such as fenoterol for breathing problems
• Digoxin - used for a fast heart beat or heart failure
If you are not sure if any of the above apply to you, talk to your doctor or pharmacist before using Combivent UDVs.
Operations
Some gases used in operations (anaesthetic gases) may affect how your medicine works. If you are about to have surgery, make sure you mention that you are taking Combivent UDVs to the doctor, dentist or anaesthetist.
Tests
If you have to provide a urine sample as part of a routine sport drug test, tell the person giving the test that you are taking this medicine. This is because Combivent UDVs contains salbutamol and this may lead to a positive result.
Pregnancy and breast-feeding
Do not use Combivent UDVs if you are pregnant, likely to get pregnant or are breast-feeding.
Driving and using machines
You may feel dizzy, or have difficulty in focusing, or blurred vision whilst taking Combivent UDVs. If this happens do not drive or use tools or machines.
3. How to use Combivent UDVs
Always use Combivent UDVs exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure. Follow these instructions to get the best results. If anything is unclear after reading this leaflet, ask your doctor, pharmacist or practice nurse.
How many dose units
The usual dose is the contents of 1 single dose unit, three or four times a day for:
Adults (including the elderly) and children over 12 years.
Combivent UDVs is not recommended for children under 12 years.
Do not swallow or give this medicine by injection.
Do not use more than your doctor has told you
See your doctor straight away if:
• You feel that your medicine is not working as well as usual
• You need to use the nebuliser more than your doctor has recommended.
Your doctor may need to check how well your medicine is working.
In some cases your doctor may need to change your medicine.
How to use your nebuliser
Read through numbers 1 to 6 first, before starting to use your nebuliser.
1. Get your nebuliser ready by following the manufacturer's instructions. Ask your doctor if you are not sure how to use it.
2.
• Open the pouch and remove the strip of unit dose vials.
• Carefully separate a new dose unit from the strip
• Do not use if it is already open or if the liquid inside is discoloured.
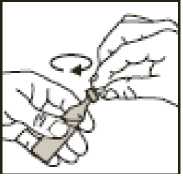
3.
• Twist off the top
• Always hold it upright while you do this
4.
• Squeeze all the contents of the dose unit into the nebuliser chamber
• Your doctor will tell you if you need to use a different amount
• If your doctor has told you that your medicine needs to be diluted, you will be given ‘sterile sodium chloride 0.9%' solution. Your doctor will tell you how to do this
5. Use your nebuliser as directed by your doctor.
6. • After you have finished, dispose of any leftover medicine carefully
• Follow the manufacturer's instructions on how to clean your nebuliser
• It is important to keep your nebuliser clean.
If any of the liquid or mist accidentally gets into your eyes you may get painful, stinging or red eyes, dilated pupils, blurred vision, see colours or lights. If this happens, talk to your doctor for advice. If you get problems with your eyes at any other time, talk to your doctor for advice.
If you use more Combivent UDVs than you should
If you use more of this medicine than you should, talk to a doctor or go to a hospital straight away.
If you forget to take Combivent UDVs
• If you forget a dose, take it as soon as you remember it.
• However, if it is time for the next dose, skip the missed dose.
• Do not take a double dose to make up for a forgotten dose.
If you have any further questions, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Combivent UDVs can cause side effects, although not everybody gets them.
Tell your doctor straight away if you notice any of the following serious side effects - you may need urgent medical treatment:
• If after taking Combivent UDVs you are wheezy or have other difficulties in breathing, do not take any more (unless you have been told to by your doctor).
• Allergic reactions - the signs may include skin rash, itching and nettle rash. In severe cases the signs include swelling of your tongue, lips and face, sudden difficulties in breathing and a fall in your blood pressure that may cause dizziness.
See your doctor straight away if you have any of these side effects.
The side effects described below have been experienced by people taking Combivent UDVs.
Uncommon (affects less than 1 in 100 people)
• Feeling nervous, shaky or dizzy
• Dry mouth
• Cough, headache
• Feeling sick (nausea)
• Throat irritation
• Increase in blood pressure
• Increased heart rate or uneven heart beats (palpitations)
• Voice problems (‘dysphonia')
• Skin reaction
Rare (affects less than 1 in 1,000 people)
• Irregular heart beat
• Regular but abnormally fast heart rate (supraventricular tachycardia)
• Chest pain (due to heart problems such as angina). Tell your doctor or pharmacist if this occurs but do not stop taking this medicine unless told to do so
• Blurred vision, dilated pupils, glaucoma, painful, stinging, or red eyes, swelling of the eyes, see colours or lights
• Increased sweating
• Unexpected tightening of the chest immediately after inhaling the medicine
• Dry throat, swelling of the throat
• Difficulty in breathing or speaking due to a brief spasm of your vocal muscles
• Diarrhoea, constipation, being sick (vomiting) or other problems with your digestive system
• Inflammation of the mouth
• Muscle cramps, muscle weakness and pain
• Difficulty in passing water (urine)
• Feeling weak
• Fall in blood pressure
• Mood changes
You may also get unusually low levels of potassium in your blood (called ‘hypokalemia’). If this happens, your doctor will keep checking your potassium levels.
If any of the liquid or mist accidentally gets into your eyes you may get painful, stinging or red eyes, dilated pupils, blurred vision, see colours or lights. If this happens, talk to your doctor for advice. If you get problems with your eyes at any other time, talk to your doctor for advice.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Combivent UDVs
Keep out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and the vial label.
The expiry date refers to the last day of the month.
Do not store above 25°C. Do not refrigerate or freeze. Do not use if the liquid is discoloured. Keep the vials in the outer carton in order to protect from light.
6. Further Information
Combivent UDVs contain a nebuliser solution. Each single dose unit contains ipratropium bromide 500 micrograms and salbutamol 2.5 mg (as sulphate) in 2.5 ml of aqueous solution.
Also contains sodium chloride, (1 N) hydrochloric acid and purified water.
What Combivent UDVs looks like and contents of the pack
Combivent UDVs are a Low density polyethylene vial containing aqueous clear solution.
Your medicine must be used with a nebuliser. This changes Combivent into a mist for you to breathe in. Each single dose unit contains a clear, colourless solution.
Combivent UDVs are available in packs of 60 units.
Manufactured by: Laboratoire Unither, France. Procured from within the EU. Product Licence holder: Quadrant Pharmaceuticals Ltd. Lynstock House, Lynstock Way, Lostock, Bolton BL6 4SA. Repackaged by Maxearn Ltd, Bolton BL6 4SA.
Combivent UDVs Nebuliser Solution PL 20774/1188
Combivent is a registered trademark of Boehringer Ingelheim Pharma GmbH & Co. KG.
Date of preparation 21st January 2015
PP3/1188/V2
POM