Dacepton 10Mg/Ml Solution For Injection/Infusion
Out of date information, search anotherPACKAGE LEAFLET: INFORMATION FOR THE USER
Dacepton 10 mg/ml Solution for injection or infusion
Apomorphine hydrochloride hemihydrate
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
The name of your medicine is Dacepton 10 mg/ml Solution for injection or infusion, which will be referred to as Dacepton 10 mg/ml throughout this leaflet.
In this leaflet:
1. What Dacepton 10 mg/ml is and what it is used for
2. Before you use Dacepton 10 mg/ml
3. How to use Dacepton 10 mg/ml
4. Possible side effects
5. How to store Dacepton 10 mg/ml
6. Further information
1. WHAT Dacepton 10 mg/ml IS AND WHAT IT IS USED FOR
Dacepton 10 mg/ml contains apomorphine solution for injection. It is injected into the area under the skin (subcutaneously). The active ingredient in Dacepton 10 mg/ml is apomorphine hydrochloride hemihydrate. There is 10 mg of apomorphine hydrochloride hemihydrate in each millilitre of solution.
Apomorphine hydrochloride belongs to a group of medicines known as dopamine agonists. Dacepton 10 mg/ml is used to treat Parkinson’s disease. Apomorphine helps to reduce the amount of time spent in an ‘off’ or immobile state in people who have previously been treated for Parkinson’s disease with levodopa (another treatment for Parkinson‘s disease) and/or other dopamine agonists.
Your doctor or nurse will help you to recognise the signs of when to use your medicine.
Despite the name, apomorphine does not contain morphine.
2. BEFORE YOU USE Dacepton 10 mg/ml
Do NOT use Dacepton 10 mg/ml
- if you are under 18 years of age
- if you have breathing difficulties
- if you have dementia or Alzheimer’s disease
- if you suffer from a mental illness with symptoms such as hallucinations, delusions, disordered thoughts, loss of contact with reality
- if you have liver problems
- if you have severe dyskinesia (involuntary movements) or severe dystonia (inability to move) despite taking levodopa
- if you are allergic to apomorphine or any of the other ingredients of Dacepton 10 mg/ml (for other ingredients, see Section 6).
- if you or someone in your family are known to have an abnormality of electrocardiogram (ECG) called "long QT syndrome”. Tell your doctor.
Take special care with Dacepton 10 mg/ml Please inform your doctor, nurse or pharmacist:
- if you have kidney problems
- if you have lung problems
- if you have heart problems
- if you have low blood pressure or feel faint and dizzy when you stand
- if you are taking any medicines to treat high blood pressure
- if you feel sick or suffer from being sick
- if your Parkinson‘s disease causes certain mental problems such as hallucinations and confusion
- if you are elderly or frail
Tell your doctor if you or your family/carer notices that you are developing urges or cravings to behave in ways that are unusual for you and you cannot resist the impulse, drive or temptation to carry out certain activities that could harm yourself or others. These are called impulse control disorders and can include behaviours such as addictive gambling, excessive eating or spending, an abnormally high sex drive or an increase in sexual thoughts or feelings. Your doctor may need to adjust or stop your dose.
Check with your doctor or pharmacist before taking your medicine if:
You are using medicines that are known to affect the way your heart beats. This includes medicines used for heart rhythm problems (such as quinidine and amiodarone), for depression (including tricyclic antidepressants such as amitriptyline and imipramine) and for bacterial infections (‘macrolide’ antibiotics such as erythromycin, azithromycin and clarithomycin) and domperidone.
Taking other medicines
If you use Dacepton 10 mg/ml with other medicines the effect of those medicines may be altered.
This is especially true for:
- Medicines such as clozapine to treat some mental disorders
- Medicines to lower your blood pressure
- Other medicines for Parkinson‘s disease
Your doctor will tell you if you need to change the dose of your apomorphine or any of your other medicines.
If you are taking levodopa (another medicine for Parkinson’s disease) as well as apomorphine your doctor should check your blood regularly.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Using Dacepton 10 mg/ml with food and drink
Food and drink do not affect the way Dacepton 10 mg/ml will work.
Pregnancy and breast-feeding
Dacepton 10 mg/ml should not be used during pregnancy unless clearly necessary. Check with your doctor or nurse before using Dacepton 10 mg/ml if you are pregnant, think you may be pregnant or you are planning to become pregnant.
It is not known whether Dacepton 10 mg/ml is transferred to breast milk. Talk to your doctor if you are breast-feeding or intend to breast-feed. Your doctor will explain to you, whether you should continue/discontinue breast-feeding or continue/discontinue taking this medicine.
Ask your doctor or pharmacist for advice before using any medicine.
Driving and using machines
Dacepton 10 mg/ml can cause drowsiness and a strong desire to sleep. Do not drive or use any tools or machinery if Dacepton 10 mg/ml affects you in this way.
Information about the ingredients of Dacepton 10 mg/ml
Dacepton 10 mg/ml contains sodium metabisulphite which rarely can cause a severe allergic reaction with symptoms such as rash or itchy skin, difficulty breathing, puffiness of the eyelids, face or lips, swelling or redness of the tongue. If you experience these side effects, immediately go to the nearest hospital casualty department.
Dacepton 10 mg/ml contains less than 1 mmol (23mg) of sodium per 10 ml, i.e. essentially sodium-free.
3. HOW TO USE DACEPTON 10 MG/ML
Always use Dacepton 10 mg/ml exactly as your doctor has told you. You should check with your doctor, nurse or pharmacist if you are not sure.
Domperidone should be taken at least 2 days before Dacepton 10 mg/ml is started to stop you feeling or being sick.
Do not use Dacepton 10 mg/ml if
- The solution has turned green.
- The solution is cloudy or you can see particles in it.
Where to inject your Dacepton 10 mg/ml
Inject your Dacepton 10 mg/ml into an area under the skin (subcutaneously) as shown by your doctor or nurse.
Do not inject Dacepton 10 mg/ml into a vein
How much to use
The amount of Dacepton 10 mg/ml you should use and the number of injections required each day will depend upon your personal needs. Your doctor will discuss this with you and tell you how much of your medicine you should inject and how often.
The amount that will work best for you will have been determined during your visit to the specialist clinic.
- The usual daily dose is between 3 mg and 30 mg.
- You may need as much as 100 mg per day.
- Typically, you will need between 1 and 10 injections each day
- Each injection should not be more than 10 mg per hour.
If our symptoms are not controlled well enough with separate injections or if you find you are requiring more than 10 injections per day, you may require a continuous infusion of apomorphine. Your doctor or nurse will decide if you need this.
For a continuous infusion:
- The usual dose is between 1 mg and 4 mg per hour.
- Usually this is given to you when you are awake and stopped before you go to sleep.
- A different site for each infusion should be used every 12 hours.
The choice of which minipump and/or syringe driver to use will be decided by your doctor.
You should check with your doctor, nurse or pharmacist if you are not sure.
What you need to inject Dacepton 10 mg/ml
For an injection, you will need:
- One syringe and needle
- A bin called a ‘Sharps’ bin to dispose safely of the used needles and glass containers. These are available from your doctor or pharmacist. Alternatively, use another suitable container such as an empty coffee jar.
How to open Dacepton 10 mg/ml
Ampoules with a single spot (blue break point):
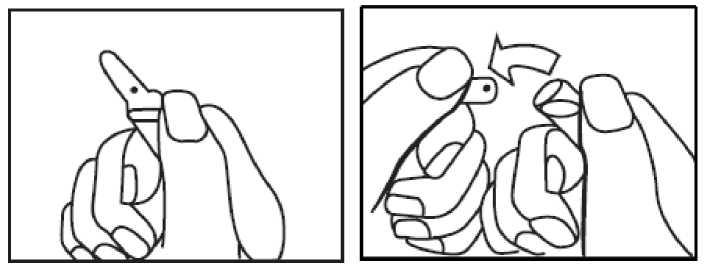
- Locate the spot positioned directly above the short score mark on the thin part of the neck. This score is the breaking point of the ampoule.
- Hold the bottom of the ampoule in one hand.
- Cover the spot with your thumb and use your forefinger to grasp the neck of the ampoule as shown in the diagram.
- Apply pressure with your thumb covering the spot in a backward direction.
- Carefully dispose of the top of the ampoule in a ‘Sharps‘ bin.
Once opened Dacepton 10 mg/ml should be used immediately.
Injecting Dacepton 10 mg/ml
- Place the needle firmly on the end of the syringe.
- Withdraw the volume you require for your dose as advised by your doctor or nurse.
- You may need to dilute Dacepton 10 mg/ml before use. Your doctor or nurse will have told you if you need to do this and how to do it.
- Inject your medicine as shown by your doctor or nurse into an area under the skin (subcutaneously).
- Discard used syringes, needles and ampoules in a "Sharps" bin (available from your doctor or pharmacist) or other suitable container, such as an empty coffee jar.
- Take care not to splash any of the solution onto yourself or the carpet as it may stain green.
If you have any further questions about the use of this medicine, ask your doctor or nurse.
If you use more Dacepton 10 mg/ml than you should
- Tell your doctor or contact your nearest hospital emergency department immediately.
- You may experience a slow heart rate, excessive sickness, excessive sleepiness and/or difficulty breathing. You may also feel faint or dizzy particularly when you stand up, due to low blood pressure. Lying down and raising your feet may help you to feel better.
If you forget to use Dacepton 10 mg/ml
Take it when you next require it. Do not take a double dose to make up for a forgotten dose.
If you stop using Dacepton 10 mg/ml
Do not stop using Dacepton 10 mg/ml without first talking with your doctor.
If you have any further questions on the use of this medicine, ask your doctor, nurse or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Dacepton 10 mg/ml can cause side effects, although not everybody gets them.
If you experience an allergic reaction stop taking Dacepton 10 mg/ml and contact a doctor or your nearest hospital emergency department immediately.
The signs of an allergic reaction may include:
- rash
- breathing difficulties
- swelling of the face, lips, throat or tongue
Dacepton 10 mg/ml may sometimes cause the following:
Very common side effects (affect more than 1 in 10 people):
- Lumps under the skin at the site of injection which are sore, troublesome and may be red and itchy. In order to avoid getting these lumps, it is advisable to change the site of injection every time you insert the needle.
Common side effects (affect less than1 in 10 people):
- Feeling sick or being sick, particularly when starting Dacepton 10 mg/ml. lf you are taking domperidone and still feel sick, or if you are not taking domperidone and you feel sick, tell your doctor or nurse as soon as possible
- Feeling tired or extremely sleepy
- Confusion or hallucinations
- Yawning
- Feeling dizzy or light-headed when standing up
Uncommon side effects (affect less than 1 in 100 people):
- Increased involuntary movements or increased shakiness during ‘on‘ periods
- Haemolytic anaemia, an abnormal breakdown of red blood cells in the blood vessels or elsewhere in the body. This is an uncommon side effect that can occur in patients also taking levodopa.
- Rashes
- Breathing difficulties
- Injection site ulceration
- Reduction in red blood cells which can make the skin pale yellow and cause weakness or breathlessness
- Reduction in blood platelets, which increases the risk of bleeding or bruising
Rare side effects (affect less than 1 in 1000 people):
- An allergic reaction
- Eosinophilia, an abnormally high amount of white blood cells in the blood or in body tissues.
Side effects occurring in an unknown number of users:
- Swelling of the legs, feet or fingers
- inability to resist the impulse, drive or temptation to perform an action that could be harmful to you or others, which may include:
o Strong impulse to gamble excessively despite serious personal or family consequences.
o Altered or increased sexual interest and behaviour of significant concern to you or to others, for example, an increased sexual drive. o Uncontrollable excessive shopping or spending o binge eating (eating large amounts of food in a short time period) or compulsive eating (eating more food than normal and more than is needed to satisfy your hunger)
Tell your doctor if you experience any of these behaviors; she or he will discuss ways of managing or reducing the symptoms
If you get any side effects, talk to your doctor, nurse or pharmacist. This includes any side effects not listed in this leaflet.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE DACEPTON 10 MG/ML
Keep out of the reach and sight of children.
Keep the ampoules in the outer carton in order to protect from light.
Do not refrigerate or freeze.
Chemical and physical in-use stability has been demonstrated for up to 24 hours at 15 - 25°C when the product is diluted with sodium chloride 9 mg/ml (0.9%).
From a microbiological point of view the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2°C to 8°C, unless opening and dilution has taken place in controlled and validated aseptic conditions.
Do not use Dacepton 10 mg/ml after the expiry date which is stated on the carton and label after "EXP.”. The expiry date refers to the last day of that month.
Once you have opened the ampoule, use it immediately.
Do not use if the solution has turned green. It should only be used if the solution is clear, colourless to slightly yellow and free of particles.
Used syringes, needles and ampoules should be discarded in a ‘Sharps’ bin or other suitable container, such as an empty coffee jar. When your ‘Sharps’ bin or container is full, please give it to your doctor or pharmacist for safe disposal.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. FURTHER INFORMATION What Dacepton 10 mg/ml contains
The active substance is apomorphine hydrochloride hemihydrate. Each millilitre of Dacepton 10 mg/ml contains 10 mg of apomorphine hydrochloride hemihydrate.
Dacepton 10 mg/ml is available in 5 ml ampoules containing 50 mg of apomorphine hydrochloride hemihydrate.
Dacepton 10 mg/ml also contains:
- Sodium metabisulphite (E223)
- Hydrochloric acid (or sodium hydroxide)
- Water for Injections
Refer to ’Section 2:Information about the ingredients of Dacepton 10 mg/ml’ regarding sodium metabisulphite.
What Dacepton 10 mg/ml looks like and contents of the pack
Dacepton 10 mg/ml is a clear and colourless to slightly yellow solution for injection or infusion.
Glass ampoules containing 5ml Solution for injection or infusion, in packs of 1, 5 or 10 ampoules.
Bundle packs: 5 x 1, 10 x 1,2 x 5, 5 x 5, 10 x 5, 3 x 10 and 10 x 10
Not all pack sizes may be marketed.
Marketing Authorisation Holder
EVER Neuro Pharma Oberburgau 3 4866 Unterach Austria
Manufacturer
EVER Neuro Pharma Oberburgau 3 4866 Unterach Austria
This medicinal product is authorised in the Member States of the EEA under the following names:
Austria
Belgium
Bulgaria
Czech Republic
Denmark
Estonia
Finland
France
Germany
Greece
Hungary
Ireland
Italy
Latvia
Lithuania
The Netherlands
Norway
Poland
Portugal
Romania
Dacepton® 10 mg/ml Injektions-/Infusionslosung Dacepton® 10 mg/ml oplossing voor injectie/infusie Dacepton® 10 mg/ml MHwe^MOHeH/uH$y3MOHeH pa3TBop Dacepton® 10 mg/ml Injekcnf/infuzm roztok Dacepton® 10 mg/ml injektionsv^ske/infusionsv^ske Dacepton® 10 mg/ml
Dacepton® 10 mg/ml injektioneste/infuusioneste Dopaceptin® 10 mg/ml solution injectable/pour perfusion Dacepton® 10 mg/ml Injektions-/Infusionslosung Dopaceptin® 10 mg/ml Evsoigo SidAuga/AidAuga Yia SYXuap Dacepton® 10 mg/ml oldatos injekcio / infuzio Dacepton® 10 mg/ml solution for injection / infusion Dacepton®
Dacepton® 10 mg/ml skTdums injekcijam / infuzijam Dacepton® 10 mg/ml Injekcinis / infuzinis tirpalas Dacepton® 10 mg/ml oplossing voor injectie / infusie Dacepton® 10 mg/ml injeksjonsv^ske / infusjonsv^ske Dacepton®
Dacepton® 10 mg/ml Solugao injectavel ou para perfusao Dacepton® 10 mg/ml Solute injectabila/perfuzabila
Sweden Dacepton® 10 mg/ml injektionsvatska/infusionsvatska
Slovak republic Dacepton® 10 mg/ml Injekcny a infuzny roztok Slovenia Dacepton® 10 mg/ml raztopina za injiciranje/infundiranje
Spain Dacepton® 10 mg/ml Solucion inyectable y para perfusion
This leaflet was last approved in {MM/YYYY}
03/2013