Decapeptyl Sr 11.25Mg Powder For Suspension For Injection
Front Page



Package leaflet: Information for the user
* Decapeptyl® SR 11.25 mg
powder for suspension for injection
Triptorelin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, please ask your doctor, pharmacist or nurse.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Decapeptyl SR 11.25 mg is and what it is used for
2. What you need to know before you use Decapeptyl SR 11.25 mg
3. How to use Decapeptyl SR 11.25 mg
4. Possible side effects
5. How to store Decapeptyl SR 11.25 mg
6. Contents of the pack and other information
1. What Decapeptyl SR 11.25 mg is and what it is used for
The active ingredient in Decapeptyl SR 11.25 mg is triptorelin. Triptorelin belongs to a group of medicines called gonadotropin releasing hormone (GnRH) agonists. Triptorelin is similar to the gonadotropin releasing hormone which occurs naturally in your body.
In men, triptorelin lowers the levels of the hormone testosterone.
In women, it reduces oestrogen levels.
Decapeptyl SR 11.25 mg has three different uses. It is used in men, women and children to treat completely different conditions.
Decapeptyl SR is available in two other strengths: Decapeptyl SR 3 mg is used once a month and Decapeptyl SR 22.5 mg is used once every 6 months. Not all dose strengths are approved for all indications. Askyour doctor if you would like to discuss changing your treatment.
This leaflet gives information for all three uses of Decapeptyl SR 11.25 mg. Please read all the sections that are about you and your condition.
MEN
In men, Decapeptyl SR 11.25 mg is used to treat prostate cancer.
WOMEN
In women, Decapeptyl SR 11.25 mg is used to treat Endometriosis - a condition in which the tissue that normally lines the uterus (endometrium) grows in other places.
CHILDREN
In children, Decapeptyl SR 11.25 mg is used to treat Puberty that occurs at a very young age (Precocious Puberty). This is called 'early puberty' in the rest of this leaflet.
2. What you need to know before you use Decapeptyl SR 11.25 mg MEN
Do not use Decapeptyl SR 11.25 mg:
• If you are allergic to triptorelin or similar types of drugs (other GnRH agonists) or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions:
• There have been reports of depression in patients taking Decapeptyl SR 11.25 mg which may be severe. If you are taking Decapeptyl SR 11.25 mg and develop depressed mood, inform your doctor.
• If you are using medicines for delaying your normal blood clotting, you may experience bruising at the site of injection.
• In adults if triptorelin or other GnRH agonists are used for a long period of time you may increase the risk of developing thin or weak bones, especially if you are a heavy drinker, a smoker, have a family history of osteoporosis (a condition that affects the strength of your bones), have a poor diet or take anticonvulsants (medicines for epilepsy or fits) or corticosteroids (steroids). If you have anything wrong with you that affects your bones, such as osteoporosis, tell your doctor. This may affect the way your doctor decides to treat you.
• When you first start treatment with Decapeptyl SR 11.25 mg it actually increases the level of your hormones for a short time. This means that you may feel worse to begin with (see section 4'Possible side effects'for more information). The doctor may give you some medicine (an antiandrogen) to prevent your symptoms from getting worse. After a short time the amount of hormone will drop and your symptoms will get better.
• During the first weeks of treatment, Decapeptyl SR 11.25 mg may, in isolated cases, and in common with other GnRH agonists, cause the spinal cord to compress or the urethra (where you pass urine) to block. You will be monitored by your doctor and given treatment for these conditions if they occur.
• Tell your doctor if you have diabetes.
• Tell your doctor if you have any heart or blood vessel conditions, including heart rhythm problems (arrhythmia), or are being treated with medicines for these conditions. The risk of heart rhythm problems may be increased when using Decapeptyl SR 11.25 mg.
• If you have an enlargement (benign tumour) of the pituitary gland that you were unaware of, this may be discovered during treatment. Symptoms include sudden headache, problems with eyesight and paralysis of the eye muscles.
• Tell your doctor if you notice blood in your urine or if you find it difficult or painful to urinate.
• Tell your doctor if you have back pain, weakness, numbness or tingling in your legs.
Your doctor may give you another drug to help you feel better during this time.
Other medicines and Decapeptyl SR 11.25 mg:
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Decapeptyl SR 11.25 mg might interfere with some medicines used to treat heart rhythm problems (e.g. quinidine, procainamide, amiodarone and sotalol) or might increase the risk of heart rhythm problems when used with some other drugs (e.g. methadone (used for pain relief and part of drug addiction detoxification), moxifloxacin (an antibiotic), antipsychotics used for serious mental illnesses).
Drugs which increase the level of a hormone called prolactin may react with Decapeptyl SR
11.25 mg. Many different kinds of drugs may increase prolactin levels.
Driving and using machines:
You may feel dizzy, tired or have problems with your sight such as blurred vision. These are possible side effects of treatment or from the underlying disease. If you experience any of these side effects, you should not drive or use machines.
WOMEN
Do not use Decapeptyl SR 11.25 mg:
• If you are allergic to triptorelin or similar types of drugs (other GnRH agonists) or any of the other ingredients of this medicine (listed in section 6).
• If you are pregnant or breast-feeding.
Warnings and precautions:
• There have been reports of depression in patients taking Decapeptyl SR 11.25 mg which may be severe. If you are taking Decapeptyl SR 11.25 mg and develop depressed mood, inform your doctor.
• If you are using medicines for delaying your normal blood clotting, you may experience bruising
at the site of injection.
• In adults if triptorelin or other GnRH agonists are used for a long period of time you may increase the risk of developing thin or weak bones, especially if you are a heavy drinker, a smoker, have a family history of osteoporosis (a condition that affects the strength of your bones), have a poor diet or take anticonvulsants (medicines for epilepsy or fits) or corticosteroids (steroids). If you have anything wrong with you that affects your bones, such as osteoporosis, tell your doctor. This may affect the way your doctor decides to treat you.
• When you first start treatment with Decapeptyl SR 11.25 mg it actually increases the level of your hormones for a short time. This means that you may feel worse to begin with (see section 4 'Possible side effects'for more information). After a short time the amount of hormone will drop and your symptoms will get better.
• Tell your doctor if you have diabetes.
• Tell your doctor if you have any heart conditions.
■ If you have an enlargement (benign tumour) of the pituitary gland that you were unaware of, this may be discovered during treatment. Symptoms include sudden headache, problems with eyesight and paralysis of the eye muscles.
• You may have some vaginal bleeding in the first month of treatment. After that your periods normally stop.
• Tell your doctor if you have bleeding after the first month of treatment.
• Your periods should start about 5 months after the last injection.
You must use some form of contraception other than the'pill' while you are having treatment and until you start your next period. Your doctor may suggest using a barrier method of contraception such as a condom or diaphragm (cap).
Other medicines and Decapeptyl SR 11.25 mg:
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Drugs which increase the level of a hormone called prolactin may react with Decapeptyl SR
11.25 mg. Many different kinds of drugs may increase prolactin levels.
Pregnancy and breast-feeding:
Do not take Decapeptyl SR 11.25 mg if you are pregnant or breast-feeding.
Driving and using machines:
You may feel dizzy, tired or have problems with your sight such as blurred vision. These are possible side effects of treatment or from the underlying disease. If you experience any of these side effects, you should not drive or use machines.
CHILDREN
Do not use Decapeptyl SR 11.25 mg:
• If you are allergic to triptorelin or similar types of drugs (other GnRH agonists) or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions:
• There have been reports of depression in patients taking Decapeptyl SR 11.25 mg which may be severe. If you are taking Decapeptyl SR 11.25 mg and develop depressed mood, inform your doctor.
■ If you are using medicines for delaying your normal blood clotting, you may experience bruising at the site of injection.
■ If you have a progressive brain tumour, tell your doctor. This may affect the way your doctor decides to treat you.
■ Girls who have an early puberty may have some vaginal bleeding in the first month of treatment.
• Tell your doctor if you have diabetes.
• Tell your doctor if you have any heart conditions.
■ If you have an enlargement (benign tumour) of the pituitary gland that you were unaware of, this may be discovered during treatment. Symptoms include sudden headache, problems with eyesight and paralysis of the eye muscles.
Other medicines and Decapeptyl SR 11.25 mg:
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Drugs which increase the level of a hormone called prolactin may react with Decapeptyl SR
11.25 mg. Many different kinds of drugs may increase prolactin levels.
3. How to use Decapeptyl SR 11.25 mg
MEN
Decapeptyl SR 11.25 mg will be injected into a muscle, usually your bottom, by a doctor or nurse. On this leaflet there are instructions for them that explain how to prepare the injection.
You will normally receive an injection once every 3 months.
Also read 'Other medicines and Decapeptyl SR 11.25 mg' in section 2.
If you are given more Decapeptyl SR 11.25 mg than you should:
If you are given too much Decapeptyl SR 11.25 mg you may experience additional or more severe side effects (see section 4'Possible side effects').
If you forget to take a dose of Decapeptyl SR 11.25 mg:
As soon as you realise that you have missed an injection you should tell your doctor. You will then be given your next injection.
If you stop receiving Decapeptyl SR 11.25 mg:
If you stop receiving your Decapeptyl SR 11.25 mg injection before your doctor tells you to then your symptoms are likely to return.
If you have any further questions on the use of this product, ask your doctor or pharmacist. WOMEN
Decapeptyl SR 11.25 mg will be injected into a muscle, usually your bottom, by a doctor or nurse. On this leaflet there are instructions for them that explain how to prepare the injection.
You will normally receive two injections, the second one three months after the first. Each injection will be given in the first five days of your period.
Also read 'Other medicines and Decapeptyl SR 11.25 mg' in section 2.
If you are given more Decapeptyl SR 11.25 mg than you should:
If you are given too much Decapeptyl SR 11.25 mg you may experience additional or more severe side effects (see section 4'Possible side effects').
If you forget to take a dose of Decapeptyl SR 11.25 mg:
As soon as you realise that you have missed an injection you should tell your doctor. You will then be given your next injection.
If you stop receiving Decapeptyl SR 11.25 mg:
If you stop receiving your Decapeptyl SR 11.25 mg injection before your doctor tells you to then your symptoms are likely to return.
If you have any further questions on the use of this product, ask your doctor or pharmacist CHILDREN
Decapeptyl SR 11.25 mg will be injected into a muscle, usually your bottom, by a doctor or nurse. On this leaflet there are instructions for them that explain how to prepare the injection.
You will normally receive an injection once every 3 months.
Your doctor will decide when treatment should be stopped (normally when you are about 12 if you are a girl and about 13-14 if you are a boy).
Also read 'Other medicines and Decapeptyl SR 11.25 mg' in section 2.
If you are given more Decapeptyl SR 11.25 mg than you should:
If you are given too much Decapeptyl SR 11.25 mg you may experience additional or more severe side effects (see section 4'Possible side effects').
If you forget to take a dose of Decapeptyl SR 11.25 mg:
As soon as you realise that you have missed an injection you should tell your doctor. You will then be given your next injection.
If you stop receiving Decapeptyl SR 11.25 mg:
If you stop receiving your Decapeptyl SR 11.25 mg injection before your doctor tells you to then your symptoms are likely to return.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Decapeptyl SR 11.25 mg can have side effects although not everybody gets them.
In rare cases you may experience a severe allergic reaction. Tell your doctor immediately if you develop symptoms such as swallowing or breathing problems, a rash, swelling of your lips, face, throat or tongue.
MEN
Many of the side effects are expected, due to the change in the level of testosterone in your body. These effects include hot flushes, impotence and decreased libido.
Side effects which are very common (may affect more than 1 in 10 people) are hot flushes, weakness, excessive sweating, back pain, pins and needles sensation in the legs, reduced libido and impotence.
Side effects which are common (may affect up to 1 in 10 people) are nausea, dry mouth, pain, bruising, redness and swelling at injection site, muscle and bone pain, pain in the arms and legs, oedema (build-up of fluid in the body tissues), lower abdominal pain, high blood pressure, allergic reaction, increase in weight, dizziness, headache, loss of libido, depression and mood changes.
Side effects which are uncommon (may affect up to 1 in 100 people) are increase of blood platelets, feeling your heartbeat, ringing in the ears, vertigo, blurred vision, pain in abdomen, constipation, diarrhoea, vomiting, drowsiness, severe shivering associated with sweating and a fever, sleepiness, pain, some blood tests affected (including raised liver function tests), blood pressure increased, weight loss, loss of appetite, increase of appetite, gout (severe pain and swelling in the joints usually in the big toe), diabetes, excessive lipids in the blood, joint pain, muscle cramp, muscle weakness, muscle pain, swelling and tenderness, bone pain, tingling or numbness, inability to sleep, feeling of irritability, development of enlarged breasts in men, breast pain, reduction in testicular size, pain in testicles, difficulty in breathing, acne, hair loss, itching, rash, redness of skin, hives waking up to pass urine, problems passing urine and nosebleeds.
Side effects which are rare (may affect up to 1 in 1,000 people) are red or purple discolorations on the skin, abnormal sensation in the eye, blurring or disturbance in vision, sensation of fullness in the abdomen, flatulence, abnormal sense of taste, chest pain, difficulty in standing, flu-like symptoms, fever, anaphylactic reaction (serious allergic reaction which can cause dizziness or difficulty in breathing), inflammation of the nose/ throat increased body temperature, stiff joints, joint swelling, musculoskeletal stiffness, osteoarthritis, memory loss, feeling confused, decreased activity, having a feeling of elation, shortness of breath when lying flat, blisters and low blood pressure.
During post-marketing surveillance the following side effects have also been reported: changes in ECG (QT prolongation), anaphylactic reaction (serious allergic reaction which

Decapeptyl-Signes-11.25_mg-Leaflet-UK-1043140-(3.1).indd 1
1043140-MOCK UP!
16/11/15 14:08
causes difficulty in breathing or dizziness), general discomfort, anxiety, rapid formation of wheals due to swelling of the skin or mucous membranes and urinary incontinence.
As with other GnRH agonists, an increase in white blood cell count may be found in patients being treated with Decapeptyl SR 11.25 mg.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.oov.uk/yellowcard By reporting side effects, you can help provide more information on the safety of this medicine.
WOMEN
Many of the side effects are expected due to the change in the level of oestrogens in your body.
These very common side effects (may affect more than 1 in 10 people) include headache, decreased libido, mood swings, difficulty in sleeping, breast disorder, ovarian hyperstimulation syndrome, pain during or after sexual intercourse, painful periods, genital bleeding, pelvic pain, dryness of the vagina, excessive sweating, acne, oily skin and hot flushes.
Side effects which are common (may affect up to 1 in 10 people) are breast pain, muscle cramps, painful joints, weight gain, feeling sick, depression, nervousness, abdominal pain or discomfort, pain, bruising, redness and swelling at injection site, swelling and tenderness, allergic reaction, pain in the arms and legs, dizziness.
Side effects which are uncommon (may affect up to 1 in 100 people) are feeling your hearbeat, vertigo, dry eye, blurred vision, bloating, vomiting, diarrhoea, dry mouth, flatulence, mouth ulcer, weight decrease, decrease in appetite, water retention, back pain, muscle pain, abnormal taste, loss of sensations, temporary loss of consciousness, memory loss, lack of concentration, tingling or numbness, involuntary muscle movement, mood change, anxiety, disorientation, bleeding after sex, prolapse, irregular period, painful period and heavy period, small cysts (swelling) on the ovaries which can cause pain, discharge from the vagina, difficulty breathing, nosebleed, hair loss, dry skin, excessive bodily hair, brittle nails, itching, hives and skin rash.
During post-marketing surveillance the following side effects have also been reported: general discomfort, increased blood pressure, increased body temperature, anaphylactic reaction (serious allergic reaction which causes difficulty in breathing or dizziness), some blood tests affected (including raised liver function tests) muscle weakness, confusion, absence of menstrual periods, rapid formation of wheals due to swelling of the skin or mucous membranes, abnormal sensations in the eyes and/or changes in sight.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.gov.uk/vellowcard
By reporting side effects, you can help provide more information on the safety of this medicine.
CHILDREN
Side effects which are very common (may affect more than 1 in 10 people) include vaginal bleeding which may occur in girls in the first month of treatment.
Side effects which are common (may affect up to 1 in 10 people) include pain in abdomen, pain bruising, redness and swelling at injection site, headache, hot flushes, weight gain, acne, hypersensitivity reactions.
Side effects which are uncommon (may affect up to 1 in 100 people) are blurred vision, vomiting, constipation, nausea, general discomfort, overweight, neck pain, changes in mood, pain in breast, nosebleeds, itching, rash or hives in the skin.
During post-marketing surveillance the following side effects have also been reported: high blood pressure, abnormal vision, anaphylactic reaction (serious allergic reaction which causes difficulty in breathing or dizziness) seen in adult men and women, some blood tests affected include hormone levels, rapid formation of wheals due to swelling of
the skin or mucous membranes, muscle pain, mood disorders, depression, nervousness.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at www.mhra.aov.uk/vellowcard
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Decapeptyl SR 11.25 mg
Keep this medicine out of the sight and reach of children.
Do not use the vial or ampoule after the expiry date printed on the box.
This medicine should not be stored above 25°C. The vial and ampoule should be kept in the outer box.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information What Decapeptyl SR 11.25 mg contains:
The active substance of Decapeptyl SR 11.25 mg is triptorelin. Each vial contains sufficient quantity of triptorelin (as triptorelin acetate) to ensure that the minimum triptorelin quantity injected is 11.25 mg.
The other ingredients are D,L lactide-glycolide copolymer, mannitol, carmellose sodium, polysorbate 80.
What Decapeptyl SR 11.25 mg looks like and contents of the pack
Each pack contains:
1 clear glass vial with a rubber stopper and an aluminium cap containing the powder 1 glass ampoule containing the suspension vehicle
1 syringe
2 needles.
Marketing Authorisation Holder
Ipsen Limited, 190 Bath Road, Slough, Berkshire, SL1 3XE, UK.
Manufacturer
Ipsen Pharma Biotech, Signes, France.
Is this leaflet hard to see or read? Please phone +44 (0) 1753 627777 and ask for help.
This leaflet was last revised in November 2015.
The following information is intended for medical or healthcare professionals only: INSTRUCTIONS FOR RECONSTITUTION
1. PREPARATION OF THE PATIENT BEFORE RECONSTITUTION
■ Prepare the patient by disinfecting the injection site. This operation needs to be performed first because once reconstituted, the drug should be injected immediately.
2. PREPARATION OF THE INJECTION
Two needles are provided in the box:
• Needle 1: a 20G needle (38 mm of length) without safety device to be used for reconstitution
• Needle 2: a 20G needle (38 mm of length) with safety device to be used for injection
Needle 1 -38 mm

Needle 2-38 mm

The presence of bubbles on top of the lyophilisate is a normal appearance of the product.
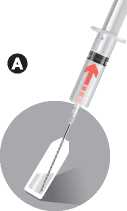

Take out the ampoule containing the solvent. Tap any solution within the tip of the ampoule back to the main body of the ampoule.
Screw Needle 1 (without safety device) on to the syringe. Do not remove the needle protection yet.
Break open the ampoule with dot face up.
Remove the needle protection from Needle 1. Insert the needle in the ampoule and draw up all the solvent into the syringe.
Put aside the syringe containing the solvent.
Take out the vial containing the powder. Tap any powder which has accumulated at the top of the vial back to the bottom of the vial.
Remove the plastic tab on top of the vial.
Take back the syringe containing the solvent, and insert the needle through the rubber stopper vertically into the vial. Inject the solvent slowly, so that, if possible, it washes down the entire upper part of the vial.

Pull up Needle 1 above the liquid level. Do not remove the needle from the vial. Reconstitute the suspension, by swirling gently from side to side. Do not invert the vial.
Continue swirling long enough to obtain a homogeneous and milky suspension.
Important: Check there is no unsuspended powder in the vial (if any powder clumps are present, continue swirling until they disappear).
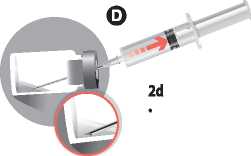
When the suspension is homogeneous, pull down the needle and without inverting the vial, draw up all of the suspension. A small amount will remain in the vial and should be discarded. An overfill Is included to allow for this loss.
Grasp the coloured hub to disconnect the needle. Remove Needle 1 used for the reconstitution from the syringe. Screw on to the syringe Needle 2.
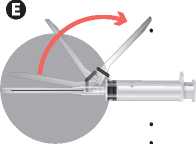
Move the safety sheath away from the needle and towards the syringe barrel. The safety sheath remains in the position you set.
Remove the needle protection from the needle.
Prime the needle to remove air from the syringe and inject immediately.
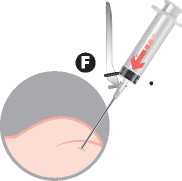
To avoid precipitation, inject immediately into the gluteal muscle previously disinfected.
4. AFTER USE
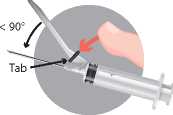
• Activation of the safety system using a one-handed technique.
• Note: Keep your finger behind the tab at all times.
There are two alternatives to activate the safety system:
• Method A: push the tab forward with your finger
Method B: push the sheath to a flat surface
Press firmly
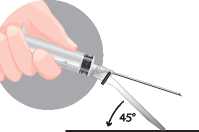
Method B
In both cases press down with a firm quick motion until a distinct audible click is heard. Visually confirm that the needle is fully engaged under the lock.
Lock

Used needles, any unused suspension or other waste materials should be disposed of in accordance with local requirements.
^ IP5EN ((
0459
Decapeptyl-Signes-11.25_mg-Leaflet-UK-1043140-(3.1).indd 2 1043140-MOCK UP 1
16/11/15 14:08