Deltius 10 000 I.u./Ml Oral Drops Solution
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
DELTIUS 10 000 I.U. /ml oral drops, solution
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
1 ml oral solution (50 drops) contains 10 000 I.U. cholecalciferol (vitamin D3), equivalent to 0.25 mg.
1 drop contains 200 I.U. cholecalciferol (vitamin D3)
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Oral drops, solution.
Clear and colorless to greenish-yellow oily solution without visible solid particles
and/or precipitate
4. CLINICAL PARTICULARS
4.1 Therapeutic indications
■ Prophylaxis of rickets and osteomalacia in children and adults
■ Prophylaxis of rickets in preterm newborns
■ Prophylaxis of vitamin D deficiency in children and adults with an identified risk
■ Prophylaxis of vitamin D deficiency in children and adults with malabsorption
■ Treatment of rickets and osteomalacia in children and adults
4.2 Posology and method of administration
1 drop contains 200 IU vitamin D3.
• Paediatric posology
- prevention of deficiency 0-1 years 400 IU/day (2 drops)
- prevention of deficiency 1-18 years 600 IU/day (3 drops)
- doses of up to 1000 IU/day (5 drops) may be required to prevent deficiency in some children
- treatment of deficiency 0-18 years 2000 IU/day (10 drops) for 6 weeks, followed by maintenance therapy of 400-1000 IU/day (2 - 5 drops).
• Pregnancy and breast-feeding posology
- prevention of deficiency 400 IU/day (2 drops)
- doses of up to 2000 IU/day (10 drops) may be required to prevent deficiency in some women (see below)
- even higher doses may be required during breast-feeding if women choose not to give the infant a vitamin D3 supplement
• Adult posology
- prevention of vitamin D3 deficiency 600 - 800 IU/day (3 - 4 drops). Higher doses may be required in certain situations, see below.
- as an adjunct to specific therapy for osteoporosis: 800 IU/day (4 drops)
• Certain populations are at high risk of vitamin D3 deficiency, and may require higher doses and monitoring of serum 25(OH)D:
- Institutionalised or hospitalised individuals
- Dark skinned individuals
- Individuals with limited effective sun exposure due to protective clothing or consistent use of sun screens
- Obese individuals
- Patients being evaluated for osteoporosis
- Use of certain concomitant medications (eg, anticonvulsant medications, glucocorticoids)
- Pregnant women
- Patients with malabsorption, including inflammatory bowel disease and coeliac disease
- Those recently treated for vitamin D3 deficiency, and requiring maintenance therapy.
The Recommended Tolerable Upper Intake Levels of vitamin D3 are as follows:
Adults, including pregnant and lactating women 4000 IU/day; children and adolescents aged 11-17 years 4000 IU/day; children aged 1-10 years 2000 IU/day; infants aged 0-1 years 1000 IU/day.
• Special Populations
- Patients with renal impairment: no specific adjustment is required
- Other conditions: in obese patients, patients with malabsorption syndromes, and patients on medications affecting vitamin D3 metabolism, higher doses are required for the treatment and prevention of vitamin D3 deficiency (2 - 3 times higher).
• Method of administration
Patients should be advised to take DELTIUS preferably with a meal (see section
5.2 Pharmacokinetic properties - “Absorption”).
The product should be shaken before use.
DELTIUS has a taste of olive oil. DELTIUS can be taken as is or to facilitate the intake it can also be mixed with a spoonful or a small amount of cold or lukewarm food immediately prior to use. The patient should be sure to take the entire dose.
In children, DELTIUS can be mixed with a small amount of children’s foods, yogurt, milk, cheese or other dairy products. The parents should be warned not to mix DELTIUS into a bottle of milk or container of soft foods in case the child does not consume the whole portion, and does not receive the full dose. The parents should ensure that their child takes the entire dose. In children who are not breast-feeding, the prescribed dose should be administered with a meal.
See also section 6.6, Special precautions for handling and disposal.
• Instructions for use
The packs contain 1 bottle and a dropper applicator cap. The bottle is sealed by a plastic childproof cap. The dropper is protected by a plastic cylinder. To use, follow the instructions below:
a. To Open the bottle, press down and twist the cap at the same time (see Figure 1);
b. Before using the dropper applicator cap for the first time, twist off the plastic cylinder by unscrewing the plastic cylinder stem the dropper applicator cap (see Figure 2);
c. Insert the dropper applicator cap into the bottle to take up the contents. Collect the prescribed number of drops onto a spoon;
d. To close the bottle, carefully screw the dropper applicator cap until closed (there is no need to press down) (see Figure 3);
e. Place the medicine into the original package.
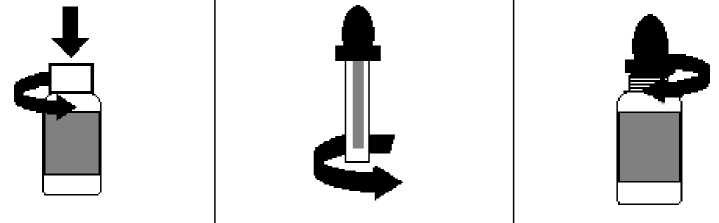
Figure 1
4.3 Contraindications
Hypersensitivity to the active substance, cholecalciferol (vitamin D3), or to any of the excipients listed in section 6.1.
Hypercalcaemia, hypercalciuria Hypervitaminosis D
Kidney stones (nephrolithiasis, nephrocalcinosis) in patients with current chronic hypercalcaemia
4.4 Special warnings and precautions for use
Vitamin D3 should be used with caution in patients with impairment of renal function and the effect on calcium and phosphate levels should be monitored. The risk of soft tissue calcification should be taken into account.
Caution is required in patients receiving treatment for cardiovascular disease (see section 4.5 Interaction with other medicinal products and other forms of interaction - cardiac glycosides including digitalis).
DELTIUS should be prescribed with caution in patients with sarcoidosis, due to a possible increase in the metabolism of vitamin D3 in its active form. In these patients the serum and urinary calcium levels should be monitored.
Allowances should be made for the total dose of vitamin D3 in cases associated with treatments already containing vitamin D3, foods enriched with vitamin D3, cases using milk enriched with vitamin D3, and the patient’s level of sun exposure.
There is no clear evidence for causation between vitamin D3 supplementation and renal stones, but the risk is plausible, especially in the context of concomitant calcium supplementation. The need for additional calcium supplementation should be considered for individual patients. Calcium supplements should be given under close medical supervision.
Oral administration of high-dose vitamin D3 (500,000 IU by single annual bolus) was reported to result in an increased risk of fractures in elderly subjects, with the greatest increase occurring during the first 3 months after dosing.
During long-term treatment with a daily dose exceeding 1,000 IU vitamin D3 the serum calcium values must be monitored.
4.5 Interactions with other medicinal products and other forms of interaction
Concomitant use of anticonvulsants (such as phenytoin) or barbiturates (and possibly other drugs that induce hepatic enzymes) may reduce the effect of vitamin D3 by metabolic inactivation.
In cases of treatment with thiazide diuretics, which decrease urinary elimination of calcium, monitoring of serum calcium concentration is recommended.
Concomitant use of glucocorticoids can decrease the effect of vitamin D3.
In cases of treatment with drugs containing digitalis and other cardiac glycosides, the administration of vitamin D3 may increase the risk of digitalis toxicity (arrhythmia). Strict medical supervision is needed, together with serum calcium concentration and electrocardiographic monitoring if necessary.
Simultaneous treatment with ion exchange resin such as cholestyramine, colestipol hydrochloride, orlistat or laxative such as paraffin oil, may reduce the gastrointestinal absorption of vitamin D3.
The cytotoxic agent actinomycin and imidazole antifungal agents interfere with vitamin D3 activity by inhibiting the conversion of 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 by the kidney enzyme, 25-hydroxyvitamin D-1-hydroxylase.
4.6 Fertility, pregnancy and lactation
Pregnancy
There are no or limited amount of data from the use of cholecalciferol (vitamin D3) in pregnant women. Studies in animals have shown reproductive toxicity (see section 5.3 Preclinical safety data). The recommended daily intake for pregnant women is 400 IU, however, in women who are considered to be vitamin D3 deficient a higher dose may be required (up to 2 000 IU/day - 10 drops). During pregnancy women should follow the advice of their medical practitioner as their requirements may vary depending on the severity of their disease and their response to treatment vitamin D3 and its metabolites are excreted in breast milk.
Breast-feeding
Vitamin D3 can be prescribed while the patient is breast-feeding if necessary. This supplementation does not replace the administration of vitamin D3 in the neonate.
Overdose in infants induced by nursing mothers has not been observed, however, when prescribing additional vitamin D3 to a breast-fed child the practitioner should consider the dose of any additional vitamin D3 given to the mother.
4.7 Effects on ability to drive and use machines
There are no data on the effects of DELTIUS on the ability to drive. However, an effect on this ability is unlikely.
4.8 Undesirable effects
Adverse reactions are listed below, by system organ class and frequency. Frequencies are defined as: uncommon (>1/1,000, <1/100) or rare (>1/10,000, <1/1,000).
Metabolism and nutrition disorders Uncommon: Hypercalcaemia and hypercalciuria Skin and subcutaneous disorders:
Rare: pruritus, rash, and urticaria.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
4.9 Overdose
Discontinue DELTIUS when calcaemia exceeds 10.6 mg/dl (2.65 mmol/l) or if the calciuria exceeds 300 mg/24 hours in adults or 4-6 mg/kg/day in children. An overdose manifests as hypercalcaemia and hypercalciuria, the symptoms of which include the following: nausea, vomiting, thirst, constipation, polyuria, polydipsia and dehydration.
Chronic overdosage may lead to vascular and organ calcification, as a result of hypercalcaemia.
Treatment in cases of overdose
Discontinue administration of DELTIUS and initiate rehydration.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: vitamin D3 and analogues, cholecalciferol ATC Code: A11CC05
In its biologically active form, vitamin D3 stimulates intestinal calcium absorption, incorporation of calcium into the osteoid, and release of calcium from bone tissue. In the small intestine it promotes rapid and delayed calcium uptake. The passive and active transport of phosphate is also stimulated. In the kidney, it inhibits the excretion of calcium and phosphate by promoting tubular resorption. The production of parathyroid hormone (PTH) in the parathyroids is inhibited directly by the biologically active form of vitamin D3. PTH secretion is inhibited additionally by the increased calcium uptake in the small intestine under the influence of biologically active vitamin D3.
5.2 Pharmacokinetic properties
The pharmacokinetics of vitamin D3 is well known.
Absorption
Vitamin D3 is well absorbed from the gastro-intestinal tract in the presence of bile, so the administration with the major meal of the day might therefore facilitate the absorption of vitamin D3.
Distribution and biotransformation
It is hydroxylated in the liver to form 25-hydroxy-cholecalciferol and then undergoes further hydroxylation in the kidney to form the active metabolite 1,25-dihydroxy-cholecal ciferol (calcitriol).
Elimination
The metabolites circulate in the blood bound to a specific a - globin, vitamin D3 and its metabolites are excreted mainly in the bile and faeces.
Characteristics in Specific Groups of Subjects or Patients
A 57% lower metabolic clearance rate is reported in subjects with renal impairment as compared with that of healthy volunteers.
Decreased absorption and increased elimination of vitamin D3 occurs in subjects with malabsorption.
Obese subjects are less able to maintain vitamin D3 levels with sun exposure, and are likely to require larger oral doses of vitamin D3 to replace deficits.
5.3 Preclinical safety data
Pre-clinical studies conducted in various animal species have demonstrated that toxic effects occur in animals at doses much higher than those required for therapeutic use in humans.
In toxicity studies at repeated doses, the effects most commonly reported were increased calciuria and decreased phosphaturia and proteinuria.
Hypercalcaemia has been reported in high doses. In a state of prolonged hypercalcaemia, histological alterations (calcification) were more frequently borne by the kidneys, heart, aorta, testes, thymus and intestinal mucosa. Cholecalciferol (vitamin D3) has been shown to be teratogenic at high doses in animals.
At doses equivalent to those used therapeutically, cholecalciferol (vitamin D3) has no teratogenic activity.
Cholecalciferol (vitamin D3) has no potential mutagenic or carcinogenic activity.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Refined olive oil.
6.2 Incompatibilities
In absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
6.3 Shelf life
3 years.
After first opening the bottle: the product may be stored for a maximum of 6 months.
6.4 Special precautions for storage
Do not store above 30° C.
Do not freeze or refrigerate
Keep the bottle in the outer carton in order to protect from light.
For storage condition after first opening of the medicinal product, see section 6.3.
6.5 Nature and contents of container
Amber glass Type III bottle of 20 ml containing 10 ml of oral drops solution (corresponding to 500 drops), sealed by a childproof polypropylene cap.
1 dropper applicator cap with a colourless type III glass stem and polypropylene cap is provided Each pack contains 1 bottle and 1 dropper applicator cap.
6.6 Special precautions for disposal and other handling
You should preferably take DELTIUS together with meal (see section 5.2 Pharmacokinetic properties - “Absorption”).
Do not store any product or food mixture that contains DELTIUS for use at a later time or a next meal (see section 4.2 Posology and method of administration).
Any unused medicinal product or waste material should be disposed of in accordance with the local requirements.
7 MARKETING AUTHORISATION HOLDER
Italfarmaco S.p.A.
Viale Fulvio Testi, 330- 20126-Milano, Italy
8 MARKETING AUTHORISATION NUMBER(S)
PL 13297/0003
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
27/08/2013
10 DATE OF REVISION OF THE TEXT
22/01/2015