Desmopressin Spray 10 Micrograms/Dose Nasal Spray Solution
Out of date information, search anotherSUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Desmopressin Spray 10 Micrograms/dose, nasal spray, solution
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
1 ml of nasal spray, solution contains:
100 micrograms desmopressin acetate trihydrate corresponding to 89 micrograms desmopressin.
One actuation of 0.1 ml delivers 10 micrograms of desmopressin acetate trihydrate.
For a full list of excipients, see section 6.1.
3. PHARMACEUTICAL FORM
Nasal spray, solution Clear colourless solution
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
- treatment of vasopressin-sensitive central diabetes insipidus
- diagnostic test of the renal concentrating capacity
4.2 Posology and method of administration
For nasal use
Before application blow the nose. Place nozzle inside the nostril and press once. One actuation delivers a dose of 10 micrograms desmopressin acetate trihydrate. If higher doses are prescribed it is recommended that one-half of the dose be administered per nostril. While spraying breathe in slightly.
- Treatment of vasopressin-sensitive central diabetes insipidus
|
Daily dose |
Number of sprays | |
|
Adults: |
10 - 20 micrograms |
1 - 2 |
|
Children and |
5 - 10 micrograms |
1 |
Adolescents < 18 years:
5 micrograms cannot be administered with Desmopressin Spray, another medicinal product containing desmopressin should be used.
The daily dose should be divided into 1 - 2 doses (in the morning and if required at bedtime).
In case of insufficient efficacy the dose can be increased on a case by case basis up to 40 pg in adults divided into 2 doses of 20 pg (2 sprays in the morning and at bedtime) and to 20 pg in children and adolescents (< 18 years) divided into 2 doses of 10 pg (1 spray in the morning and at bedtime).
The optimal dosage of Desmopressin Spray must be established individually and should be based on measurements of urine volume and osmolality. The treatment should aim at two goals: a normal water balance and an adequate duration of sleep (as a result of the improvement in the nocturia and nocturnal enuresis often observed in central diabetes insipidus). The goal of 6 to 7 dry nights/week should be achieved.
- Diagnostic test of renal concentrating capacity
|
Weight |
Intranasal dose |
Number of sprays |
|
< 10 kg |
10 micrograms |
1 |
|
10-30 kg |
20 micrograms |
2 |
|
30-50 kg |
30 micrograms |
3 |
|
> 50 kg |
40 micrograms |
4 |
The test serves both to distinguish diabetes insipidus from polyurias of other aetiology and to determine reduced renal concentrating capacity due to urinary tract infections as well as for early diagnosis of tubulo-interstitial damage e.g. due to lithium, analgesics, chemotherapeutics or immunosuppressants.
The desmopressin test is carried out preferably in the morning. Fluid intake should be restricted from 1 hour before to 8 hours after administration of the medicinal product (see Section 4.4). Children under 5 years old and patients with cardiovascular diseases or hypertension should reduce their fluid intake to 50%. It is recommended that the bladder should be emptied at the time of the administration.
Urine osmolality should be determined before and twice after administration of desmopressin. Urine collected within the first hour should be discarded. Urine osmolality is determined in the two subsequent urine samples, preferentially taken two and four hours after administration of desmopressin.
In order to determine the renal concentrating capacity, the higher value is compared to the baseline value or to an age-specific reference value.
A substantial rise in urine osmolality along with a significant decrease in urine volume is indicative of central diabetes insipidus. Low values, absence of a rise or only a slight rise in urinary osmolality indicate reduced renal concentrating capacity.
The safety and efficacy of desmopressin in specific patient populations (with renal or hepatic impairments or other concomitant diseases) has not been investigated.
Instructions for handling:
Remove protective cap, keep bottle upright. Prime pump 3 times before the first application only until a uniform mist is achieved.
When spraying always hold the bottle in such a way that the dip tube points down and is immersed in the solution. Insert the nozzle into one of the nostrils and spray once. When a higher dose is needed, spray alternately into each nostril.
After use, replace the protective cap and store bottle upright.
4.3 Contraindications
- Hypersensitivity to desmopressin or any of the excipients.
- Primary polydipsia and polydipsia due to alcohol abuse.
- Hyponatraemia or risk of developing hyponatraemia.
- Cardiac insufficiency and other conditions requiring treatment with diuretic agents.
- syndrome of inappropriate secretion of antidiuretic hormone, because this syndrome is associated with dilutional hyponatraemia.
- Polyuria without objective diagnostic of central diabetes insipidus
- Von Willebrand disease Type IIb
- Thrombotic thrombocytopenic purpura (TTP)
4.4. Special warning and precautions for use
Desmopressin should be used with caution in patients with coronary heart disease, hypertension and severe hypertension or fluid and electrolyte imbalance (such as patients with renal impairment or cystic fibrosis patients). The use of desmopressin in patients with renal impairments may theoretically increase the risk of water retention and hyponatraemia.
Desmopressin should be used with caution in pregnant women.
Desmopressin therapy without concomitant adjustment of fluid intake may lead to fluid retention and hyponatraemia, accompanied by symptoms such as weight gain, headache, nausea and oedema. In severe cases cerebral oedema, convulsions and coma may occur.
In particular, infants and elderly patients (depending on their general health) are at increased risk of water and electrolyte imbalance.
As a precautionary measure to prevent hyperhydration and hypo-natremia, fluid intake should be reduced in conditions characterised by fluid and electrolyte imbalance or by increased intracranial pressure.
There is some evidence from post-marketing data for the occurrence of severe hyponatraemia in association with the nasal spray formulation of desmopressin, when it is used in the treatment of cranial diabetes insipidus
The risk of water intoxication and hyponatraemia can also be minimised by keeping to the recommended starting doses and by avoiding concomitant use of medicinal products which may increase the antidiuretic effect of desmopressin (see Section 4.5).
It is important to monitor body weight and blood pressure during therapy with Desmopressin Spray. An increase in body weight may be due to overdosage or, more often, due to increased fluid intake. In case of weight increase or plasma sodium level <130mmol/L or plasma osmolality < 270mOsm/kg: the fluid intake should be limited as much as possible and the administration of desmopressin should be discontinued.
Following diagnostic testing for diabetes insipidus or renal concentration capacity, care should be taken to prevent fluid overload. Fluid should not be forced, orally or parenterally, and patients should only take as much fluid as they require to satisfy thirst.
When Desmopressin Spray is used for diagnostic testing purposes fluid intake should be limited to 500 ml from 1 hour before to 8 hours after administration.
Renal concentration capacity testing in infants below the age of 1 year should only be performed under carefully supervised conditions in hospital.
Absorption may be irregular in patients with oedema, scarring or other abnormal conditions of the nasal mucosa.
4.5 Interaction with other medicinal products and other forms of interaction
Clofibrate, chlorpromazine, carbamazepine, tricyclic antidepressants, serotonin reuptake inhibitors and non steroidal antiinflammatory medicinal products (NSAIDs) may enhance the antidiuretic effect of desmopressin thus increasing the risk of water intoxication and hyponatraemia.
Glibenclamide and lithium may attenuate the antidiuretic effect of desmopressin.
Desmopressin may enhance the effect of antihypotensive and attenuate the effect of antihypertensive medicinal products.
4.6. Pregnancy and lactation
Pregnancy
Data on a limited number (n = 53) of exposed pregnancies in women with diabetes insipidus indicate rare cases of malformations in children treated during pregnancy. To date, no other relevant epidemiological data are available. Animal studies do not indicate direct or indirect harmful effects with respect to pregnancy, embryonal/foetal development, parturition or postnatal development.
Caution should be exercised when prescribing to pregnant women. Blood pressure monitoring is recommended due to the increased risk of pre-eclampsia.
Lactation
Results from analyses of milk from nursing mothers receiving high dose Desmopressin (300 micrograms intranasally) indicate that the amounts of Desmopressin that may be transferred to the child are considerably less than the amounts required to influence diuresis.
4.7 Effects on ability to drive and use machines
No studies on the effect of Desmopressin Spray on the ability to drive and use machines have been performed.
Desmopressin has no known effect on the ability to drive and use machines.
4.8. Undesirable effects
The following undesirable effects of desmopressin were recorded from clinical studies and postmarketing experience. Adverse reactions are listed according to the following categories:
Very common: Common: Uncommon: Rare:
Very rare:
> 1/10
> 1/100 < 1/10 > 1/1,000 < 1/100 > 1/ 10,000 < 1/1,000 < 1/10,000, including isolated reports
Nervous system disorder:
Uncommon: headache Rare: cerebral oedema
Very rare: emotional disturbance in case of nocturnal enuresis
Eye disorders: Common: conjunctivitis
Respiratory, thoracic and mediastinal disorders:
Uncommon: nasal congestion, epistaxis, rhinitis
Gastrointestinal disorders:
Uncommon: nausea, abdominal cramps, vomiting
General disorders and administration site conditions:
Common: asthenia
Very rare: allergic and hypersensitivity reactions (e.g. pruritus, exanthema, fever, bronchospasms, anaphylaxis).
Metabolism disorders:
Rare: hyponatraemia
Cardiovascular/Vascular disorders:
Due to increased water reabsorption blood pressure may rise and in some cases hypertension may develop. In patients with coronary heart disease angina pectoris may occur.
These adverse effects, except for allergic reactions, may be prevented or disappear if the desmopressin dose is reduced.
4.9 Overdose
An overdose prolongs the antidiuretic effect and subsequently increases the risk of hyperhydration. Therefore, symptoms such as increase in body weight, headache, nausea, gastrointestinal cramps and in severe cases cerebral oedema, generalized convulsions and coma may be expected.
There is no antidote for desmopressin. Treatment of overdose consists of discontinuation of Desmopressin Spray and restriction of fluid intake until serum sodium is normalised. In cases of extensive overdose with the risk of water intoxication administration of a diuretic such as furosemide with concomitant monitoring of serum electrolytes should be considered. All cases of suspected cerebral oedema require immediate admission for intensive care measures.
5. PHARMACOLOGICAL PROPERTIES
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Posterior pituitary lobe hormones, Vasopressin and analogues.
ATC code: H01B A02
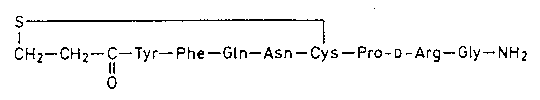
Desmopressin is a synthetic analogue of the natural human neurohypophyseal hormone L-arginine vasopressin from which it differs chemically in that the amino group of the cysteine in position 1 is removed and L-arginine has been substituted by the stereoisomeric D-arginine. As a consequence of these changes, the vasopressor action of the molecule is largely lost, whereas the antidiuretic action is enhanced and prolonged many times.
In the distal renal tubules and collecting ducts of the kidneys, desmopressin increases the permeability for water and thus water reabsorption from the primary urine.
5.2. Pharmacokinetic properties
After intranasal administration of desmopressin, the maximum plasma concentration is attained after about 50 minutes.
The plasma half-life is 2 - 3 hours. Desmopressin is excreted via the kidneys. After intranasal administration, the systemic bioavailability of desmopressin is approximately 10% of the dose administered.
The antidiuretic effect already begins after 15 minutes. Depending on the dose, it is sustained for 6 - 24 hours.
The apparent distribution volume of desmopressin is relatively small: about 0,2 l/kg bodyweight, which suggests that the peptide is not distributed in the intracellular compartment. It has been shown that desmopressin does not pass the blood-brain-barrier.
5.3. Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, genotoxicity and toxicity to reproduction.
Impairment of renal function, with a rise in serum creatinine as well as hyaline degeneration of tubule epithelia, has been demonstrated in rats at a daily dose of 47.4 micrograms /kg body weight, i.e. at exposures considered sufficiently in excess of the maximum human exposure. The alterations were reversible after termination of desmopressin treatment.
Investigations on the carcinogenic properties are not available.
6. PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Citric acid, monohydrate Disodium phosphate dihydrate Sodium chloride
Purified water.
6.2. Incompatibilities
Not applicable.
6.3. Shelf life
2 years
Shelf life after first opening:
4 weeks
6.4 Special precautions for storage
Store in the original package.
Do not store above 25°C.
Store in upright position.
6.5 Nature and contents of container
Type I amber glass bottle fitted with a metering pump composed of polypropylene, polyethylene and thermoplastic components secured with a snap on system.
Pack sizes: 6 ml.
6.6. Special precautions for disposal
No special requirements.
Any unused product or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
PH&T S.p.A Via Marostica, 1 20146 Milan - Italy
8. MARKETING AUTHORISATION NUMBER
PL 14998/0004
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
08/04/2007
10 DATE OF REVISION OF THE TEXT
04/01/2012