Dexamethasone Alapis 0.4 Mg/ Ml Oral Solution
PACKAGE LEAFLET: INFORMATION FOR THE USER
<Dexamethasone Alapis> 0.4 mg/ ml Oral Solution Dexamethasone
Read all of this leaflet carefully before you start taking this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1.
2.
3.
4.
5.
6.
What <Dexamethasone Alapis> is and what it is used for Before you take <Dexamethasone Alapis>
How to take <Dexamethasone Alapis>
Possible side-effects
How to store <Dexamethasone Alapis>
Further information
1. WHAT <Dexamethasone Alapis> IS AND WHAT IT IS USED FOR
<Dexamethasone Alapis> belongs to a group of medicines called steroids. Their full name is corticosteroids. These corticosteroids occur naturally in the body, and help to maintain health and well-being. Boosting your body with extra corticosteroid (such as <Dexamethasone Alapis>) is an effective way to treat various illnesses involving inflammation in the body. <Dexamethasone Alapis> reduces this inflammation, which could otherwise go on making your condition worse. You must take this medicine regularly to get maximum benefit from it.
<Dexamethasone Alapis> is used for one of the following:
• where natural corticosteroid levels have been reduced and you need replacement therapy
• in certain cases where swelling of the brain has occurred
• if you are having diagnostic tests for diseases which may have an effect on natural corticosteroid production as is Cushing’s syndrome (disorder of the hormonal system).
• to reduce inflammation and suppress the immune system in a variety of conditions, in particular:
- allergy (hypersensitivity)
- Polymyalgia rheumatica (chronic inflammation of the larger arteries), Polyarteritis nodosa (chronic inflammation of small and medium arteries)
- blood disorders including haemolytic anaemia (disorder which breaks down red blood cells), leukaemia (cancer of the blood), myeloma (bone marrow tumour)
-Crohn’s disease, ulcerative colitis (inflammation of the bowel particularly the rectum), hepatitis
- Polymyositis (inflammation of many muscles)
- increased pressure in the head not linked to tumours, worsening of multiple sclerosis
- inflammation of the eye
- inflammation of the kidney
- breathing problems including chronic bronchial asthma and chronic obstructive pulmonary disease (COPD) which may show as shortness of breath during exercise, difficulty breathing in and out deeply and persistent cough. Disorders where there is inflammation of the lung.
- Rheumatoid arthritis (painful joint disease), rheumatism, inflammation of a wide area of the body
- chronic and severe diseases of the skin (including Stevens- Johnson syndrome and a rare condition known as mycosis fungoides)
-Leukaemia of the lymphatic system, Hodgkins and Non-Hodgkins Disease breast cancer that has spread around the body, Kahlers disease (cancer of blood cells) and high calcium levels caused by this disease
- Following organ transplants and to prevent nausea and vomiting following chemotherapy
2. BEFORE YOU TAKE <Dexamethasone Alapis>
Do not take <Dexamethasone Alapis>:
• if you are allergic (hypersensitive) to dexamethasone or any of the other ingredients of <Dexamethasone Alapis> or you have ever had an unusual reaction to these substances
• if you have an infection that affects the whole body
• if you are pregnant, planning to become pregnant or breast feeding
• if you have a stomach or duodenal ulcer
• if you have an infection with tropical worms
Take special care with <Dexamethasone Alapis>
Before treatment with <Dexamethasone Alapis> check with your doctor:
• If you have ever had severe depression or manic depression (bipolar disorder). This includes having had depression before or while taking steroid medicines like dexamethasone.
• If any of your close family has had these illnesses.
Mental problems while taking <Dexamethasone Alapis>
Mental health problems can happen while taking steroids like <Dexamethasone Alapis>.
• These illnesses can be serious
• Usually they start within a few days or weeks of starting the medicine
• They are more likely to happen at high doses
• Most of these problems go away if the dose is lowered or the medicine is stopped. However, if problems do happen, they might need treatment.
Talk to a doctor if you (or someone taking this medicine), show any signs of mental problems. This is particularly important if you are depressed, or might be thinking about suicide. In a few cases, mental problems have happened when doses are being lowered or stopped.
Please inform any doctor, dentist or person who may be giving you treatment that you are currently taking steroids or have taken them in the past.
You should consult your doctor before taking this medicine if:
• you have kidney or liver problems
• you have high blood pressure, heart disease or you have recently had a heart attack
• you have diabetes or there is a family history of diabetes
• you have osteoporosis (thinning of the bones), particularly if you are a female who has been through the menopause
• you have suffered from muscle weakness with this or other steroids in the past
• you have glaucoma (raised eye pressure) or there is a family history of glaucoma
• you have myasthenia gravis (a condition causing weak muscles)
• you have a bowel disorder or a stomach (peptic) ulcer
• you have psychiatric problems or you have had a psychiatric illness which was made worse by this type of medicine
• you have epilepsy (condition where you have repeated fits or convulsions)
• you have migraines
•
• you have an underactive thyroid gland
• you have a parasitic infection
• you have tuberculosis, septicaemia or a fungal infection in the eye
• you have cerebral malaria
• you have herpes (cold sores or genital herpes)
• you have asthma
If you develop an infection whilst on this medicine you should talk to your doctor.
Note It is important that whilst you are taking this medicine you avoid contact with anybody who has chickenpox, shingles or measles. If you think you may have had exposure to any of these diseases, you should consult your doctor immediately. You should
also inform your doctor if you have ever had infectious diseases such as measles or chickenpox and of any vaccinations.
If you have an accident, are ill, or require any surgery (even at the dentists) or you require a vaccination (particularly with ‘live virus’ vaccines) whilst taking or when you have finished taking <Dexamethasone Alapis>, you should inform the person treating you that you are taking or have taken steroids.
If you have suppression tests (test for the amount of hormone in the body) or test for infection you should inform the person performing the test that you are taking dexamethasone as it may interfere with the results.
If a child is taking this medicine, it is important that the doctor monitors their growth and development at frequent intervals.
You may also find that your doctor will reduce the amount of salt in your diet and give you a potassium supplement whilst you are taking this medicine.
Taking other medicines
If you are taking any of the following medicines, you should consult your doctor before taking dexamethasone:
• Anticoagulant medicines which thin the blood (e.g. warfarin)
• Aspirin or similar (Non-Steroidal Anti-Inflammatory drugs) e.g. indometacin
• Medicines used to treat diabetes
• Medicines used to treat high blood pressure
• Diuretics (water tablets)
• Amphotericin B injection
• Phenytoin, Carbamazepine, Primidone (epilepsy medication)
• Rifabutin, Rifampicin (antibiotics used to treat tuberculosis)
• Antacids - particularly those containing magnesium trisilicate
• Barbiturates (medication used to aid sleep and relieve anxiety)
• Aminoglutethimide (anti-cancer treatment)
• Carbenoxolone (used in the treatment of stomach ulcers)
• Ephedrine (nasal decongestant)
• Acetazolamide (used for glaucoma and epilepsy)
• Hydrocortisone, cortisone and other corticosteroids
• Ketoconazole (for fungal infections)
• Ritonavir (for HIV)
• Antibiotics including erythromycin
• Medicines that help muscle movement in myasthenia gravis (e.g. neostigmine)
• Colestyramine (for high cholesterol levels)
• Estrogen hormones including the contraceptive pill
• Tetracosactide used in the test for adrenocortical function
• Sultopride used to calm emotions
• Ciclosporin used to prevent rejection after transplants
• Thalidomide
• Praziquantel given for certain worm infections
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before taking any medicine.
<Dexamethasone Alapis> should be prescribed during pregnancy and particularly in the first trimester only if the benefit outweighs the risks for the mother and child. If you become pregnant during the use of the product, do not stop using <Dexamethasone Alapis>, but tell your doctor immediately that you are pregnant.
Dexamethasone is excreted in breast milk. There are no known risks to infants. Nevertheless, breastfeeding should be discontinued when using higher doses or longterm treatment.
Driving and using machines <Dexamethasone Alapis> has no influence on your ability to drive or use machines.
Important information about some of the ingredients of <Dexamethasone Alapis>
If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicinal product.
<Dexamethasone Alapis> contains these kinds of sugar:
• 0.14 g sorbitol in each ml. When taken according to the dosage recommendations each dose supplies up to 3.15 g of sorbitol.
• 0.275 g maltitol in each ml. When taken according to the dosage recommendations each dose supplies up to 6.2 g of maltitol.
<Dexamethasone Alapis> contains 0.09 g propylene glycol in each ml. When taken according to the dosage recommendations each dose supplies up to 2 g of propylene glycol.
<Dexamethasone Alapis> contains small amounts of ethanol (alcohol), less than 100mg per dose.
(5.51 mg ethanol / 9 mg dexamethasone).
3. HOW TO TAKE <Dexamethasone Alapis>
<Dexamethasone Alapis> is only to be taken by mouth. Take this medicine exactly as directed by the doctor. These instructions will have been added to the dispensing label by your pharmacist.
Do not exceed or take less than the stated dose.
Do not take it more or less often than prescribed.
Always take <Dexamethasone Alapis> exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
The usual dose is:
Adults: initially 0.5 - 9mg daily in divided doses depending upon the severity of your condition then a maintenance dose of 1.5mg daily.
Children: a single dose on alternate days.
If <Dexamethasone Alapis>is being given to you as part of some hospital tests, the range given will be:
0.5 mg to 2mg per dose for a short period of time.
Do not take it more or less often than prescribed Instructions for use
Push the plastic screw cap down while turning it
Counterclockwise and remove the unscrewed cap.
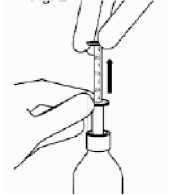
Insert the syringe into the bottle.
While holding the bottom ring, pull the top ring up to the mark which corresponds to the dose in milliliters (ml) prescribed by your doctor.
Holding the bottom ring, remove the syringe from the bottle and wipe the body of the syringe with a tissue to clean from the solution excess.
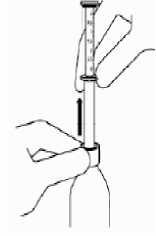
Empty its contents directly in the mouth by pressing the plunger down.
Close the bottle with the plastic screw cap.
Wash the syringe with water and leave it to air dry
If you take more <Dexamethasone Alapis> than you should
Do not exceed or take less than the stated dose.
If you take too much medicine a doctor or hospital should be contacted immediately. If you forget to take <Dexamethasone Alapis>
If you forget to take a dose, take it as soon as you remember unless it is almost time for the next one then carry on as before.
Do not take a double dose to make up for a forgotten dose.
If you stop taking <Dexamethasone Alapis>
It can be dangerous to stop taking <Dexamethasone Alapis>abruptly. If your treatment is to be stopped follow your doctor’s advice. He may tell you to reduce the amount of medicine you are taking gradually until you stop taking it altogether. The symptoms that have been reported when treatment has been stopped too quickly have included low blood pressure and in some cases, relapse of the disease for which the treatment was given.
A ‘withdrawal syndrome’ may also occur which includes fever, muscle and joint pain, inflammation of the nose lining (rhinitis), weight loss, itchy skin and inflammation of the eye (conjunctivitis). Your doctor should gradually reduce the dose.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE-EFFECTS
Like all medicines, <Dexamethasone Alapis> can cause side effects, although not everybody gets them.
Tell a doctor straight away if you experience serious mental health problems. They can affect about 5 in every 100 people taking medicines like dexamethasone. These problems include:
• feeling depressed, including thinking about suicide
• feeling high (mania) or moods that go up and down
• feeling anxious, having problems sleeping, difficulty in thinking or being confused and losing your memory
• feeling, seeing or hearing things that do not exist. Having strange and frightening thoughts, changing how you act or having feelings of being alone.
Other side effects may be:
• a bad reaction to the medicine (which may show as a rash and swelling and in severe cases difficulty in breathing)
• nausea, vomiting, hiccups, increased appetite, stomach discomfort and swollen abdomen, inflammation and ulcers in the oesophagus, peptic ulcers that may split and bleed, inflamed pancreas (which may show as pain in the back and abdomen), tearing of the bowel particularly if you have inflammatory bowel disease, changes to the number and movement of sperm, unusual fat deposits.
• salt imbalances, water retention in the body, potassium loss due to low carbon dioxide levels (hypokalaemic alkalosis), loss of protein and calcium balance
• congestive heart failure in susceptible people, high blood pressure
• thinning of the bone with an increased risk of fractures, bone disease, ruptured tendons, muscle wasting, weakness
• thrush, greater chance of picking up infections, recurrence of tuberculosis if you have already had this infection, blood disorder due to infection
• slow wound healing, thinned delicate skin, unusual marks on the skin, bruising, redness and inflammation of the skin, stretch marks, visible swollen capillaries, acne, increased sweating, impaired reaction to skin tests, skin rash, swelling, thinning of the hair
• cataracts, increased pressure in the eye, swelling of the eye, thinning of the eye membranes, worsening of existing eye infections, protrusion of the eyeballs
• blood clots
• irregular and absence of menstrual cycles (periods), impairment of the body’s regulation of hormones, stunted growth in children and teenagers, swelling and weight gain of the body and face (Cushingoid state), development of excess body hair (particularly in women), weight gain, increased requirement for diabetic medication, change in effectiveness of the medicine following stress and trauma, surgery or illness.
• fits and worsening of epilepsy, dizziness, headache, severe unusual headache with visual disturbances linked with the withdrawal of treatment, extreme mood swings, schizophrenia (mental disorder) may become worse, depression, inability to sleep.
If any of the side effects gets serious, or if you notice any side effects not listed in this
leaflet, please tell your doctor or pharmacist.
5. HOW TO STORE <Dexamethasone Alapis>
Keep out of the reach and sight of children.
Do not store above 25°C. Do not refrigerate.
After first opening, use within 3 months.
Do not use <Dexamethasone Alapis> after the expiry date which is stated on the bottle label and carton after EXP. The expiry date refers to the last day of that month.
Do not use <Dexamethasone Alapis> if you notice solid particles to be present inside the solution.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. FURTHER INFORMATION
What <Dexamethasone Alapis> contains
The active substance is dexamethasone
Each ml of solution contains 0.4 mg of dexamethasone (as dexamethasone sodium phosphate).
The other ingredients are:
benzoic acid (E210), propylene glycol (E1520), citric acid monohydrate (E330),
liquid maltitol (E965), liquid sorbitol (non-crystallising (E420)), sodium citrate (E331), garden mint flavour (containing: Peppermint, Spearmint, Menthol, Ethyl Alcohol, purified water.
What <Dexamethasone Alapis> looks like and contents of the pack
<Dexamethasone Alapis> is a colourless to faint yellow oral solution with mint flavour.
It comes in an amber glass bottle, holding 150 ml of solution, with child resistant screw-cap, along with a 3 ml oral syringe.
Marketing Authorisation Holder
<ALAPIS S.A.
2, Aftokratoros Nikolaou str.
176 71 Athens Greece>
Manufacturer
PNG Gerolymatos S.A.
Production Site (Plant B’)
4, Asklipiou Str., 145 68 Kryoneri,
Athens, Greece
This medicinal product is authorised in the Member States of the EEA under the following names:
<To be completed nationally>