Dicycloverine Hydrochloride 10Mg/5Ml Oral Solution
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Dicycloverine Hydrochloride 10mg/5ml Oral Solution
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Dicycloverine Hydrochloride Oral Solution contains the active ingredient, dicycloverine hydrochloride.
Each 5ml solution contains 10mg dicycloverine hydrochloride.
Excipients with known effect:
Each 5ml solution contains 14.735mg of propylene glycol (E1520) and 3.696g of mixture of fructose, glucose and sucrose.
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Oral solution
Clear, light yellow to yellow solution with a strawberry flavour
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Dicycloverine Hydrochloride is a smooth muscle antispasmodic primarily indicated for treatment of functional conditions involving smooth muscle spasm of the gastrointestinal tract. The commonest of these are irritable colon (mucous colitis, spastic colon).
4.2 Posology and method of administration
Adults 5-10ml (10 - 20mg) three times daily before or after meals.
Children (2-12 years):
5ml (10mg) three times daily. Children (6 months - 2 years)
2.5-5ml (5 - 10mg) three or four times daily, 15 minutes before feeds.
Do not exceed a daily dose of 20ml (40mg).
Children (0 - 6 months)
Do not give this medicine to children under 6 months of age.
Method of administration:
► Use the 2.5-5ml double-ended spoon supplied in the pack (see below) to measure the required dose.
► Swallow the solution.
► Wash the spoon with clean water after taking every dose.
Double-ended Spoon
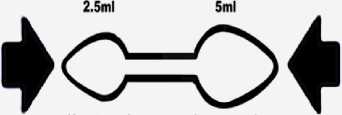
(Small end)
(Large end)
4.3 Contraindications
Known idiosyncrasy to dicycloverine hydrochloride.
Infants under 6 months of age.
This medicine contains invert sugar. Patients with rare hereditary problems of fructose intolerance or glucose galactose malabsorption should not take this medicine.
This medicine contains 3.696g of a mixture of fructose, glucose and sucrose per dose (5ml). This should be taken into account in patients with diabetes mellitus.
May be harmful to the teeth, if the medicine is intended for use for two weeks or more.
4.4 Special warnings and precautions for use
Products containing dicycloverine hydrochloride should be used with caution in any patient with or suspected of having glaucoma or prostatic hypertrophy.
Use with care in patients with hiatus hernia associated with reflux oesophagitis because anticholinergic drugs may aggravate the condition.
There are reports of infants, 3 months of age and under, administered dicycloverine hydrochloride syrup who have evidenced respiratory symptoms (breathing difficulty, shortness of breath, breathlessness, respiratory collapse, apnoea) as well as seizures, syncope, asphyxia, pulse rate fluctuations, muscular hypotonia and coma. The above symptoms have occurred within minutes of ingestion and lasted 20-30 minutes. The symptoms were reported in association with dicycloverine hydrochloride syrup therapy but the cause and effect relationship has neither been disproved or proved. The timing and nature of the reactions suggest that they were a consequence of local irritation and/or aspiration, rather than to a direct pharmacological effect. Although no causal relationship between these effects observed in infants and dicycloverine administration has been established, dicycloverine hydrochloride is contra-indicated in infants under 6 months of age.
4.5 Interaction with other medicinal products and other forms of interaction
None stated.
4.6 Fertility, pregnancy and lactation
Epidemiological studies in pregnant women with products containing dicycloverine hydrochloride (at doses up to 40mg/day) have not shown that dicycloverine hydrochloride increases the risk of foetal abnormalities if administered during the first trimester of pregnancy. Reproduction studies have been performed in rats and rabbits at doses of up to 100 times the maximum recommended dose (based on 60mg per day for an adult person) and have revealed no evidence of impaired fertility or harm to the foetus due to dicycloverine. Since the risk of teratogenicity cannot be excluded with absolute certainty for any product, the drug should be used during pregnancy only if clearly needed.
It is not known whether dicycloverine is secreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when dicycloverine is administered to a nursing mother.
4.7 Effects on ability to drive and use machines
None stated.
4.8 Undesirable effects
Side-effects seldom occur with Dicycloverine hydrochloride. However, in susceptible individuals, dry mouth, thirst and dizziness may occur. On rare occasions, fatigue, sedation, blurred vision, rash, constipation, anorexia, nausea and vomiting, headache and dysuria have also been reported.
Reporting of suspected adverse reactions:
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions at www.mhra.gov .uk/yellowcard.
4.9 Overdose
Symptoms of dicycloverine hydrochloride overdosage are headache, dizziness, nausea, dry mouth, difficulty in swallowing, dilated pupils and hot dry skin. Treatment may include emetics, gastric lavage and symptomatic therapy if indicated.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Dicycloverine hydrochloride relieves smooth muscle spasm of the gastrointestinal tract. Animal studies indicate that this action is achieved via a dual mechanism;
(1) a specific anticholinergic effect (antimuscarinic at the ACh-receptor sites) and
(2) a direct effect upon smooth muscle (musculotropic).
5.2 Pharmacokinetic properties
After a single oral 20mg dose of dicycloverine hydrochloride in volunteers, peak plasma concentration reached a mean value of 58ng/ml in 1 to 1.5 hours. 14C labelled studies demonstrated comparable bioavailability from oral and intravenous administration. The principal route of elimination is via the urine.
5.3 Preclinical safety data
None stated.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Citric acid monohydrate (E330)
Sucralose (E955)
Invert syrup (contains glucose, sucrose and fructose)
Sodium benzoate (E211)
Strawberry flavour (contains propylene glycol (E1520) and natural flavouring substances)
Purified water
6.2 Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
6.3 Shelf life
24 months.
6.4 Special precautions for storage
This medicinal product does not require any special storage conditions.
6.5 Nature and contents of container
Bottle: Amber glass
Closure: HDPE, EPE wadded, tamper evident, child resistant screw on white plastic polypropylene cap.
Dosing Device: Double ended plastic spoon with 2.5ml and 5ml measuring ends
Pack size: 100ml, 120ml, and 300ml Not all pack sizes may be marketed.
6.6 Special precautions for disposal
Any unused medicinal products or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
Syri Limited, t/a Thame Laboratories Unit 4, Bradfield Road,
Ruislip,
Middlesex,
HA4 0NU, UK
8 MARKETING AUTHORISATION NUMBER(S)
PL 39307/0019
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE
AUTHORISATION 22/01/2015
10 DATE OF REVISION OF THE TEXT
22/01/2015