Diflucan 10 Mg/Ml Powder For Oral Suspension
This medicinal product is authorised in the Member States of the EEA under the following names:
|
Austria |
Diflucan 10 mg/ml and 40 mg/ml Trockensaft |
|
Belgium, Denmark, Finland, Iceland, Ireland, Italy, Luxembourg, Norway, Portugal, Sweden, United Kingdom |
Diflucan |
|
France |
Triflucan |
|
Germany |
Diflucan Trockensaft 10 mg/ml Diflucan 40 mg/ml Pulverzur Herstellung einer Suspension zum Einnehmen |
|
Flungary |
Diflucan 10 mg/ml and 40 mg/ml por belsoleges szuszpenziohoz |
|
Greece |
Fungustatin |
|
The Netherlands |
Diflucan 10 mg/ml and 40 mg/ml |
|
Romania |
Diflucan 10 mg/ml pulbere pentru suspesie orala |
|
Slovakia |
Diflucan P.O.S. 10 mg/ml and Diflucan P.O.S. 40 mg/ml |
|
Slovenia |
Diflucan 10 mg/ml and 40 mg/ml prasekza peroralno suspenzij |
|
Spain |
Diflucan 10 mg/ml and 40 mg/ml polvo para suspension oral |
This leaflet was last revised in: UK 06/2015 IE MM/YYYY
Ref: DF 12_0
The following information is intended for healthcare professionals or for patients (where the pharmacist does not reconstitute this product):
Instructions to make up the suspension:
The reconstituted suspension will provide a white to off-white orange-flavoured suspension after reconstitution.
10 ma/ml or 40 ma/ml powder for oral suspension in 60 ml capacity bottle: 35 ml suspension after reconstitution
1. Tap the bottle to release the powder.
2. Add a small quantity of still water and shake it vigorously. Add water up to the level marked (^) on the bottle (this corresponds in total to adding the required 24ml of water).
3. Shake well for 1 to 2 minutes to obtain a well mixed suspension.
4. After reconstitution there will be a usable volume of 35ml.
5. Write the expiry date of the reconstituted suspension on the bottle label (the shelf life of the reconstituted suspension is 28 days). Any unused suspension should not be used after this date and should be returned to your pharmacist.
10 ma/ml powder for oral suspension in 175 ml capacity bottle: 100 ml suspension after reconstitution:
1. Tap the bottle to release the powder.
2. Add a small quantity of still water and shake it vigorously. Add water up to the level marked (^) on the bottle (this corresponds in total to adding the required 66ml of water).
3. Shake well for 1 to 2 minutes to obtain a well mixed suspension.
4. After reconstitution there will be a usable volume of 100ml.
5. Write the expiry date of the reconstituted suspension on the bottle label (the shelf life of the reconstituted suspension is 28 days). Any unused suspension should not be used after this date and should be returned to your pharmacist.

Package leaflet: Information for the user
Diflucan® 10 mg/ml powder for oral suspension
Diflucan® 40 mg/ml powder for oral suspension
fluconazole
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor, pharmacist or nurse.
- This medicine has been prescribed foryou only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Diflucan is and what it is used for
2. What you need to know before you take Diflucan
3. How to take Diflucan
4. Possible side effects
5. How to store Diflucan
6. Contents of the pack and other information
1. What Diflucan is and what it is used for
Diflucan is one of a group of medicines called “antifungals”. The active substance is fluconazole.
Diflucan is used to treat infections caused by fungi and may also be used to stop you from getting a candidal infection.
The most common cause of fungal infections is a yeast called Candida.
Adults
You might be given this medicine by you r doctor to treat the following types of fungal infections:
- Cryptococcal meningitis-a fungal infection in the brain
- Coccidioidomycosis- a disease of the bronchopulmonary system
- Infections caused by Candida and found in the blood stream, body organs (e.g. heart, lungs) or urinary tract
- Mucosal thrush - infection affecting the lining of the mouth, throat and denture sore mouth
- Genital thrush - infection of the vagina or penis
- Skin infections - e.g. athlete’s foot, ringworm, jock itch, nail infection
You might also be given Diflucan to:
- stop cryptococcal meningitis from coming back
- stop mucosal thrush from coming back
- reduce recurrence of vaginal thrush
- stop you from getting an infection caused by Candida (if your immune system is weak and not working properly)
Children and adolescents (0 to 17 years old)
You might be given this medicine by you r doctor to treat the following types of fungal infections:
- Mucosal thrush - infection affecting the lining of the mouth, throat
- Infections caused by Candida and found in the blood stream, body organs (e.g. heart, lungs) or urinary tract
- Cryptococcal meningitis-a fungal infection in the brain You might also be given Diflucan to:
- stop you from getting an infection caused by Candida (if your immune system is weak and not working properly).
- stop cryptococcal meningitis from coming back
2. What you need to know before you take Diflucan
Do not take Diflucan
- if you are allergic (hypersensitive) to fluconazole, to other medicines you have taken to treat fungal infections or
to any of the other ingredients of this medicine (listed in section 6). The symptoms may include itching, reddening of the skin or difficulty in breathing
- if you are taking astemizole, terfenadine (antihistamine medicines for allergies)
- if you are taking cisapride (used for stomach upsets)
- if you are taking pimozide (used for treating mental illness)
- if you are taking quinidine (used for treating heart arrhythmia)
- if you are taking erythromycin (an antibiotic for treating infections)
Warnings and precautions
Talk to your doctor or pharmacist before taking Diflucan
- if you have liver or kidney problems
- if you suffer from heart disease, including heart rhythm problems
- if you have abnormal levels of potassium, calcium or magnesium in your blood
- if you develop severe skin reactions (itching, reddening of the skin or difficulty in breathing)
Other medicines and Diflucan
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Tell your doctor immediately if you are taking astemizole, terfenadine (an antihistamine for treating allergies) or cisapride (used for stomach upsets) or pimozide (used for treating mental illness) or quinidine (used for treating heart arrhythmia) or eryth romycin (an antibiotic for treating infections) as these should not be taken with Diflucan (see section: “Do not take Diflucan if you”).
There are some medicines that may interact with Diflucan. Make sure your doctor knows if you are taking any of the following medicines:
- rifampicin or rifabutin (antibiotics for infections)
- alfentanil, fentanyl (used as anaesthetic)
- amitriptyline, nortriptyline (used as anti-depressant)
- amphotericin B, voriconazole (anti-fungal)
- medicines that thin the blood to prevent blood clots (warfarin or similar medicines)
- benzodiazepines (midazolam, triazolam or similar medicines) used to help you sleep or for anxiety
- carbamazepine, phenytoin (used for treating fits)
- nifedipine, isradipine, amlodipine, felodipine and losartan (for hypertension - high blood pressure)
- ciclosporin, everolimus, sirolimus or tacrolimus (to prevent transplant rejection)
- cyclosphosphamide, vinca alkaloids (vincristine, vinblastine or similar medicines) used for treating cancer
- halofantrine (used for treating malaria)
- statins (atorvastatin, simvastatin and fluvastatin or similar medicines) used for reducing high cholesterol levels
- methadone (used for pain)
- celecoxib, flurbiprofen, naproxen, ibuprofen, lornoxicam, meloxicam, diclofenac (Non-Steroidal Anti-Inflammatory Drugs (NSAID))
- oral contraceptives
- prednisone (steroid)
- zidovudine, also known as AZT; saquinavir (used in HIV-infected patients)
- medicines for diabetes such as chlorpropamide, glibenclamide, glipizide ortolbutamide
- theophylline (used to control asthma)
- vitamin A (nutritional supplement)
- ivacaftor (used for treating cystic fibrosis)
Diflucan with food and drink
Diflucan can betaken with or without food.
Pregnancy, breast-feeding and fertility
Ifyou are pregnant or breast-feeding, thinkyou may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine.
You should not take Diflucan while you are pregnant unless your doctor has told you to.
You can continue breast-feeding after taking a single dose of Diflucan up to 200 mg.
You should not breast-feed ifyou are taking a repeated dose of Diflucan.
|
Condition |
Dose |
|
To treat cryptococcal meningitis |
400 mg on the first day then 200 mg to 400 mg once daily for 6 to 8 weeks or longer if needed. Sometimes doses are increased up to 800 mg |
|
To stop cryptococcal meningitis from coming back |
200 mg once daily until you are told to stop |
|
To treat coccidioidomycosis |
200 mg to 400 mg once daily from 11 months for up to 24 months or longer if needed. Sometimes doses are increased up to 800 mg |
|
To treat internal fungal infections caused by Candida |
800 mg on the first day then 400 mg once daily until you are told to stop |
|
To treat mucosal infections affecting the lining of the mouth, throat and denture sore mouth |
200 mg to 400 mg on the first day then 100 mg to 200 mg until you are told to stop |
|
To treat mucosal thrush -dose depends on where the infection is located |
50 mg to 400 mg once daily for 7 to 30 days until you are told to stop |
|
To stop mucosal infections of mouth and throat from coming back |
100 mg to 200 mg once daily, or 200 mg 3 times a week, while you are at risk of getting an infection |
|
To treat genital thrush |
150 mg as a single dose |
|
To reduce recurrence of vaginal thrush |
150 mg every third day for a total of 3 doses (day 1,4 and 7) and then once a week for 6 months while you are at risk of getting an infection |
|
To treat fungal skin and nail infections |
Depending on the site of the infection 50 mg once daily, 150 mg once weekly, 300 to 400 mg once weekly for 1 to 4 weeks (Athlete’s foot may be up to 6 weeks, for nail infection treatment until infected nail is replaced) |
|
To stop you from getting an infection caused by Candida (if your immune system is weak and not working properly) |
200 mg to 400 mg once daily while you are at risk of getting an infection |
The maximum dose for children is 400 mg daily.
The dose will be based on the child’s weight in kilograms.
|
Condition |
Daily dose |
|
Mucosal thrush and throat infections caused by Candida - dose and duration depends on the severity of the infection and on where the infection is located |
3 mg per kg of body weight (6 mg per kg of body weight might be given on the first day) |
|
Cryptococcal meningitis or internal fungal infections caused by Candida |
6 mg to 12 mg per kg of body weight |
Driving and using machines
When driving vehicles or using machines it should be taken into account that occasionally dizziness or fits may occur. Diflucan powder for oral suspension contains sucrose (sugar).
- If you have an intolerance to some sugars, please contact your doctor before taking this medicine.
- Doses of 10 ml contain 5.5 g or more of sugar. This should be taken into account if you have diabetes.
- May be harmful to teeth if used for periods of longer than 2 weeks.
3. How to take Diflucan
Always take this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure. It is best to take medicine at the same time each day.
The recommended dose of this medicine for different infections are below:
Adults
Adolescents from 12 to 17 years old
Follow the dose prescribed by your doctor (either adults or children posology).
Children to 11 years old
To stop children from getting 3 mg to 12 mg per kg of body
an infection caused by weight
Candida (if their immune
system is not working
properly)
Use in children 0 to 4 weeks of age
Use in children of 3 to 4 weeks of age:
The same dose as above but given once every 2 days.
The maximum dose is 12 mg per kg of body weight every 48 hours.
Use in children less than 2 weeks old:
The same dose as above but given once every 3 days.
The maximum dose is 12 mg per kg of body weight every 72 hours.
Elderly
The usual adult dose should be given unless you have kidney problems.
Patients with kidney problems
Your doctor may change your dose, depending on your kidney function.
Instructions to make up the suspension:
It is recommended that your pharmacist makes up Diflucan powder for oral suspension before giving it to you. However where the pharmacist does not reconstitute this product, instructions are provided at the end of this leaflet in the section “The following information is intended for healthcare professionals or for patients (where the pharmacist does not reconstitute this product)”.
Instructions for use:
Shake the closed bottle of the suspension every time before using.
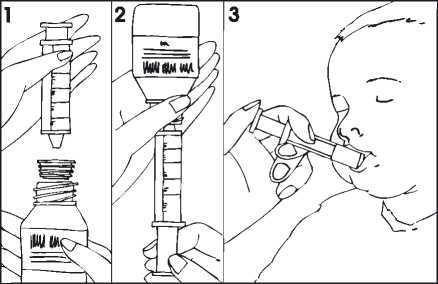
Instructions to use the paediatric syringe:
Shake the prepared suspension well.
1. Open the bottle (safety cap);
2. Insert the adapter fitted onto the syringe into the bottle neck (Figure 1);
3. Turn the bottle with the syringe upside down and withdraw the quantity of suspension prescribed by the doctor (Figure 2). The marks on the syringe are shown in ml.
The maximum dose for children is 400 mg daily (see section “3. How to take Diflucan”).
4. Remove the syringe from the bottle;
5. For younger children, the medicinal product may be given directly into the mouth from the syringe. The child should remain upright during administration. Point the syringe at the inside of the cheek; release the suspension slowly into the child’s mouth (Figure 3). For older children, the suspension may be put in a spoon and drunk by the child.
6. Rinse the syringe after use.
7. Close the bottle with the safety cap; the adapter will remain on the bottle neck.
If you take more Diflucan than you should
Taking too much Diflucan may make you unwell. Contact your doctor or your nearest hospital casualty department at once. The symptoms of a possible overdose may include hearing, seeing, feeling and thinking things that are not real (hallucination and paranoid behaviour). Symptomatic treatment (with supportive measures and gastric lavage if necessary) may be adequate.
If you forget to take Diflucan
Do not take a double dose to make up for a forgotten dose. If you forget to take a dose, take it as soon as you remember. If it is almost time for your next dose, do not take the dose that you missed.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them A few people develop allergic reactions although serious allergic reactions are rare. If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. If you get any of the following symptoms, tell your doctor immediately.
- sudden wheezing, difficulty in breathing or tightness in the chest
- swelling of eyelids, face or lips
- itching all over the body reddening of the skin or itchy red spots
- skin rash
- severe skin reactions such as a rash that causes blistering (this can affect the mouth and tongue).
Diflucan may affect your liver. The signs of liver problems include:
- tiredness
- loss of appetite
- vomiting
- yellowing of your skin or the whites of your eyes (jaundice)
If any of these happen, stop taking Diflucan and tell your doctor immediately.
Other side effects:
Additionally, if any of the following side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Common side effects (may affect up to 1 in 10 people) are:
- headache
- stomach discomfort, diarrhoea, feeling sick, vomiting
- increases in blood tests of liver function
- rash
Uncommon side effects (may affect up to 1 in 100 people) are:
- reduction in red blood cells which can make skin pale and cause weakness or breathlessness
- decreased appetite
- inability to sleep, feeling drowsy
- fit, dizziness, sensation of spinning, tingling, pricking or numbness, changes in sense of taste
- constipation, difficult digestion, wind, dry mouth
- muscle pain
- liver damage and yellowing of the skin and eyes (jaundice)
- wheals, blistering (hives), itching, increased sweating
- tiredness, general feeling of being unwell, fever
Rare side effects (may affect up to 1 in 1,000 people) are:
- lower than normal white blood cells that help defend against infections and blood cells that help to stop bleeding
- red or purple discoloration of the skin which may be caused by low platelet count, other blood cell changes
- blood chemistry changes (high blood levels of cholesterol, fats)
- low blood potassium,
- shaking
- abnormal electrocardiogram (ECG), change in heart rate or rhythm
- liver failure
- allergic reactions (sometimes severe), including widespread blistering rash and skin peeling, severe skin reactions, swelling of the lips or face
- hair loss
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly (see details below). By reporting side effects you can help provide more information on the safety of this medicine.
United Kingdom
Yellow Card Scheme website: www.mhra.gov.uk/yellowcard. Ireland
HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2; Tel: +353 1 6764971; Fax: +353 1 6762517. Website: www. hpra.ie; E-mail: medsafety@hpra.ie.
5. How to store Diflucan
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the bottle and the outer carton after EXP. The expiry date refers to the last day of that month.
Powder for oral suspension:
- Store below 25°C. Keep the bottle tightly closed.
- Once reconstituted, store the suspension below 30°C, do not freeze.
- The shelf life of the reconstituted suspension is 28 days.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information What Diflucan contains
- The active substance is fluconazole. 1 ml of reconstituted suspension contains 10 mg or 40mg fluconazole.
- The other ingredients are: sucrose, silica colloidal anhydrous, titanium dioxide (E 171), xanthan gum, sodium citrate, citric acid anhydrous, sodium benzoate and natural orange flavour (containing orange oil and maltodextrin).
What Diflucan looks like and contents of the pack:
10 mg/ml powder for oral suspension in 60 ml capacity bottle: 35 ml suspension after reconstitution 10 mg/ml powder for oral suspension in 175 ml capacity bottle: 100 ml suspension after reconstitution
- Diflucan 10 mg/ml powder for oral suspension comes in two bottle sizes
- a 60 ml capacity bottle which contains 24.4g powder. After reconstitution, the volume of the suspension is 35ml or
- a 175 ml capacity bottle which contains 67.1g powder. After reconstitution, the volume of the suspension is 100ml
- Diflucan 10 mg/ml powder for oral suspension is a dry white to off-white powder. After adding water to the powder (as instructed in this leaflet below) a white to off-white orange flavoured suspension is produced.
- In each bottle the mixture of powder and water makes 35ml or 100 ml of suspension.
- The 35ml suspension comes with a 5 ml measuring spoon and/or a 5 ml graduated syringe with a press-in bottle adaptor might also be provided to measure the correct dose.
- The 100ml suspension comes with a measuring cup to measure the correct dose.
40 mg/ml powder for oral suspension in 60 ml capacity bottle: 35 ml suspension after reconstitution
- Diflucan 40 mg/ml powder for oral suspension comes as a 60 ml capacity bottle which contains 24.4g powder. After reconstitution, the volume of the suspension is 35ml.
- Diflucan 40 mg/ml powder for oral suspension is a dry white to off-white powder. After adding water to the powder (as instructed in this leaflet below) a white to off-white orange flavoured suspension is produced.
- In each bottle the mixture of powder and water makes 35 ml of suspension.
A 5 ml measuring spoon and/or a 5 ml graduated syringe with a press-in bottle adaptor might also be provided to measure the correct dose.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Pfizer Limited Ramsgate Road Sandwich Kent
CT139NJ United Kingdom
Manufacturer
Fareva Amboise Zone Industrielle 29 route des Industries 37530 Poce-sur-Cisse France
3