Diluent For Miochol E
|
Graphics&Packing Technology Section ICN POLFA RZESZOW S.A. |
ULOTKA PIL BAUSCH 1 LOMB | |||||
|
Nazwa produktu Product Name |
Miochol-E 20mg (acetylcholine chloride) Powder and Solvent for instillation |
Kolor nadruku Colours | ||||
|
Black | | ||||||
|
Kraj Country (ISO) |
UK, IE |
Nr wykrojnika Spec No |
93336 | |||
|
Opracowane przez Designed by |
Zofia Tabisz-Mikosz Zofia.Tabisz-Mikosz@ Valeant.com phone: +48 17 865 5536 |
Kod Wytworcy Manufacturer code |
- | |||
|
Nr korekty Proof No |
3 |
Data 05.05.2014 Date |
Kod farmaceutyczny Pharmacode |
- | ||
|
Kod wersji Valeant version code |
P1UKIE01 |
Inny kod Other code |
- | |||
|
Rozmiar czcionki Font size |
minimum 7 pt |
Wymiar ulotki PIL size |
420 x 148 mm | |||
|
Kroj czcionki Font used |
Nobel BL Book Nobel BL Book italic Nobel BL Medium |
Gramatura papieru Paper weight |
- g/m2 | |||
|
Komentarze Comments (Reason for the change) | ||||||
|
PMR_ICN-14-0355-A | ||||||
|
Akceptacja Techniczna Technical Approval | ||||||
|
Akceptacja Dzialu ds. Rejestracji Regulatory Affairs Dept. Approval | ||||||
Interim Version - Not For Marketing


BAUSCH+LOMB
Miochol®-E
20mg Powder and Solvent for instillation solution for intraocular use
acetylcholine chloride
Please read this leaflet carefully before you are treated. It contains important information. Keep the leaflet in a safe place because you may want to read it again. If you have any other questions, or if there is something you don’t understand, please ask your doctor or nurse.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or nurse.
This product will usually be referred to as Miochol-E in this leaflet.
In this leaflet:
1. What Miochol-E is and what it’s used for
2. Things to consider before you are given Miochol-E
3. How Miochol-E is used
4. Possible side effects
5. How to store Miochol-E
6. Further information
1. What Miochol-E is and what it's used for
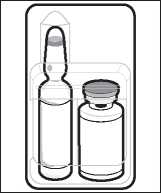
The Miochol-E pack includes a vial containing the Miochol-E powder, and an ampoule containing a liquid which is used to make up the irrigation solution. There is also a small filter hub device.
The active ingredient in Miochol-E is called acetylcholine chloride. This is one of a group of medicines called parasympathomimetics (neurohormones). It is involved with the transmission of nerve impulses in the body.
Miochol-E is used during cataract surgery and other types of eye surgery. It is used to make the pupil (at the front of the eye) contract. This helps the surgeon carry out the operation.
2. Things to consider before you are given Miochol-E
Some people MUST NOT have Miochol-E.
Talk to your doctor if:
• You think you may be allergic to acetylcholine chloride or to any of the other ingredients. (These are listed at the end of the leaflet.)
Some of the symptoms of an allergic reaction may include shortness of breath, wheezing or difficulty breathing; swelling of the face, lips, tongue or other parts of the body; rash, itching or hives on the skin.
• You are pregnant or breastfeeding.
Are you taking other medicines?
Some medicines can interfere with the way Miochol-E works. Tell your doctor or nurse if you are applying preparations containing NSAIDs (nonsteroidal anti-inflammatory drugs, e.g. ibuprofen or diclofenac) to your skin to treat pain and swelling.
Always tell your doctor or pharmacist about all the medicines you are taking. This means medicines you have bought yourself as well as medicines on prescription from your doctor.
Will there be any problems with driving or using machinery?
Your vision may be affected both by the eye surgery and by the use of Miochol-E. You should not drive or use machinery while you have any problems.
3. How Miochol-E is used
Your surgeon will work out the correct dose of Miochol-E for you. In most cases between 0.5 and 2 ml will be a suitable dose. The same dose is used both for adults and the elderly.
Your surgeon will determine when to use Miochol-E. In cataract surgery Miochol-E should only be used after the replacement lens has been inserted into the eye.
Miochol-E is applied drop by drop into the eye during surgery. (This is called intraocular irrigation.)
The solution will be made up just before it is needed by a healthcare professional. The use of Miochol-E in children has not been studied, and therefore it is not recommended.
Warning: Do not use if blister or peelable backing is damaged or broken. Open under aseptic conditions only. The contents of the unopened blister are guaranteed to be sterile.
How to prepare Miochol-E
1. Inspect unopened blister to ensure that it is intact. Peel open blister.
2. Aseptically transfer the ampoule, vial and filter hub to sterile field. Maintain asepsis during preparation of the solution.
3.
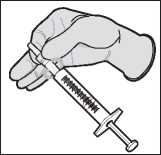
Aseptically attach a sterile 18 to 20 gauge, bevelled needle to the luer tip of a sterile disposable syringe with twisting motion to ensure a secure fit.
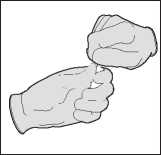
Break open the ampoule containing the solvent.
The One Point Cut (OPC) ampoule must be opened as follows:
Hold the bottom part of the ampoule with the thumb pointing to the coloured point. Grasp the top of the ampoule with the other hand, positioning the thumb at the coloured point and press back to break at the existing etched mark under the point.
Remove the needle protector and withdraw the solvent from the ampoule into the syringe. Discard the empty ampoule.
Remove and discard the plastic cap from the top of vial.
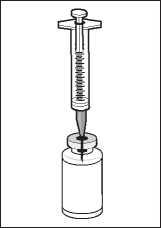
Insert the needle through the centre of the vial stopper.
Transfer the solvent from the syringe to the vial.
Shake gently to dissolve drug.
P1UKIE01
5.
6.
7.
8. 9.
|
Graphics&Packing Technology Section ICN POLFA RZESZOW S.A. |
ULOTKA PIL BAUSCH 1 LOMB | |||||
|
Nazwa produktu Product Name |
Miochol-E 20mg (acetylcholine chloride) Powder and Solvent for instillation |
Kolor nadruku Colours | ||||
|
Black | | ||||||
|
Kraj Country (ISO) |
UK, IE |
Nr wykrojnika Spec No |
93336 | |||
|
Opracowane przez Designed by |
Zofia Tabisz-Mikosz Zofia.Tabisz-Mikosz@ Valeant.com phone: +48 17 865 5536 |
Kod Wytworcy Manufacturer code |
- | |||
|
Nr korekty Proof No |
3 |
Data 05.05.2014 Date |
Kod farmaceutyczny Pharmacode |
- | ||
|
Kod wersji Valeant version code |
P1UKIE01 |
Inny kod Other code |
- | |||
|
Rozmiar czcionki Font size |
minimum 7 pt |
Wymiar ulotki PIL size |
420 x 148 mm | |||
|
Kroj czcionki Font used |
Nobel BL Book Nobel BL Book italic Nobel BL Medium |
Gramatura papieru Paper weight |
- g/m2 | |||
|
Komentarze Comments (Reason for the change) | ||||||
|
PMR_ICN-14-0355-A | ||||||
|
Akceptacja Techniczna Technical Approval | ||||||
|
Akceptacja Dzialu ds. Rejestracji Regulatory Affairs Dept. Approval | ||||||
10. Slowly withdraw the solution from the vial through the needle into the syringe.
11. Discard needle.
12. Aseptically open the filter hub pouch.
13. Aseptically attach the filter hub to the luer tip of syringe with a twisting motion to assure secure fit.
14. Aseptically attach a sterile blunt tip irrigation cannula to male luer of filter prior to intraocular irrigation.
15. Discard appropriately after use. Do not reuse the filter hub.
The solution must be
mixed just before use, since aqueous solutions of
acetylcholine are unstable. Do not use unless a clear
and colourless solution is produced.
For single use only. Discard any unused solution.
Miochol-E should not be re-sterilised. The filter
hub is recommended only for use with Miochol-E.
Aspiration through the filter is not recommended.
However, if utilised, discard needle and syringe filter
to prevent recontamination of fluids during injection.
Do not aspirate and inject through the same filter.
How to store Miochol - E
• Do not store Miochol-E above 25°C. Do not freeze.
• Do not use any Miochol-E after the expiry date printed on the box.
• Do not use any Miochol-E pack that is damaged or shows signs of tampering.
• Keep Miochol-E out of the reach and sight of children.
'li
What if you are given too much?
If you are given more Miochol-E than you need, your doctor may need to give you an injection of either atropine sulphate or adrenaline to control symptoms. Symptoms of overdose may include slow heart rate, low blood pressure, flushing, breathing difficulties and sweating.
4. Possible side effects
Miochol-E is suitable for most people, but, like all medicines, it can sometimes cause side effects.
In isolated cases it has been reported that using Miochol-E has made the cornea (the transparent layer which protects the front of the eye) become cloudy or swollen. This may affect your vision.
The following symptoms which are due to Miochol-E being absorbed into the body’s circulation from the eye, have also been reported rarely:
• slow heart rate, dizziness or lightheadedness due to low blood pressure, flushing, breathing difficulties and sweating.
If any of these side effects are severe or last for more than a few days, or if you notice anything else not mentioned here, please tell the doctor or nurse.
5. How to store Miochol-E
Keep all medicines out of the reach and sight of children.
Miochol-E should not be stored above 25°C. It should not be frozen.
It should not be used after the expiry date which is printed beside the letters "EXP" on the carton and the blister. The expiry date refers to the last day of that month.
It should not be used if the blister or peelable backing is damaged or broken.
The re-constituted solution should be used immediately.
6. Further Information
Each pack of Miochol-E contains a blister and a filtering hub device.
The blister contains a vial of white powder and an ampoule of clear, colourless solvent. The vial contains 20 mg of acetylcholine chloride as active ingredient and the inactive ingredient, mannitol. The ampoule contains 2 ml of solvent which contains sodium acetate trihydrate, magnesium chloride hexahydrate, potassium chloride, calcium acetate trihydrate, magnesium chloride hexahydrate, potassium chloride, calcium chloride dihydrate and water for injections.
The Miochol-E powder must be reconstituted with the solvent before it is used. The reconstituted solution is a clear, colourless solution providing 10 mg/ml of acetylcholine chloride (20 mg/2 ml).
The filtering hub device has a 5 micron filter and a luer lock.
Marketing Authorisation holder is Dr. Gerhard Mann Chem.-pharm. Fabrik GmbH Brunsbuettler Damm 165-173 13581 Berlin Germany
Authorisation Number:
UK - PL 13757/0017 (powder)
- PL 13757/0018 (diluent)
Republic of Ireland - PA 1245/2/1
The manufacturer responsible for release to the market is
Dr. Gerhard Mann Chem.-pharm. Fabrik GmbH Brunsbuettler Damm 165-173 13581 Berlin Germany
or
Sanofi-aventis S.p.A.
Localita Valcanello 03012 Anagni (FR)
Italy
or
Laboratoire Chauvin Avenue Jean Monnet ZI Ripotier 07200 Aubenas France
This leaflet was revised in
If you would like any more information, or would like the leaflet in a different format, please contact +44 (0) 1748 828864 (UK) or +44 (0) 1748 828849 (Republic of Ireland).
Miochol®-E is a registered trademark of Bausch & Lomb Incorporated. ©Bausch & Lomb Incorporated.
P1UKIE01
89026988
P1UKIE01_Miochol-E_20POW_2SOL__UKIE_050514_03.indd 2 2014-05-05 11:13:38