Diluent For Sandostatin Lar
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
I®) (®
Diluent for Sandostatin LAR
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
One vial contains 30 mg octreotide (as octreotide acetate)
Excipients with known effect
Contains less than 1 mmol (23 mg) sodium per dose, i.e is essentially “sodium-free”. For a full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Powder and solvent for suspension for injection.
Powder: White to white with yellowish tint.
Solvent: Clear, colourless to slightly yellow or brown solution.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
None.
4.2 Posology and method of administration
Intramuscular injection with Sandostatin LAR.
4.3 Contraindications
Hypersensitivity to any of the excipients.
4.4 Special warnings and precautions for use
General
As GH-secreting pituitary tumours may sometimes expand, causing serious complications (e.g. visual field defects), it is essential that all patients be carefully monitored. If evidence of tumour expansion appears, alternative procedures may be advisable.
The therapeutic benefits of a reduction in growth hormone (GH) levels and normalisation of insulin-like growth factor 1 (IGF-1) concentration in female acromegalic patients could potentially restore fertility. Female patients of childbearing potential should be advised to use adequate contraception if necessary during treatment with octreotide (see section 4.6).
Thyroid function should be monitored in patients receiving prolonged treatment with octreotide.
Hepatic function should be monitored during octreotide therapy.
Cardiovascular related events
Common cases of bradycardia have been reported. Dose adjustment of medicinal products such as beta blockers, calcium channel blockers, or agents to control fluid and electrolyte balance, may be necessary (see section 4.5).
Gallbladder and related events
Octreotide inhibits secretion of cholecystokinin, resulting in reduced contractility of the gallbladder and an increased risk of sludge and stone formation. Development of gallstones has been reported in 15 to 30% of long-term recipients of s.c. Sandostatin. The prevalence in the general population (aged 40 to 60 years) is about 5 to 20%. Long-term exposure to Sandostatin LAR of patients with acromegaly or gastro-entero-pancreatic tumours suggests that treatment with Sandostatin LAR does not increase the incidence of gallstone formation, compared with s.c. treatment. Ultrasonic examination of the gallbladder before and at about 6-monthly intervals during Sandostatin LAR therapy is however recommended. If gallstones do occur, they are usually asymptomatic; symptomatic stones should be treated either by dissolution therapy with bile acids or by surgery.
Glucose metabolism
Because of its inhibitory action on growth hormone, glucagon, and insulin release, Sandostatin LAR may affect glucose regulation. Post-prandial glucose tolerance may be impaired. As reported for patients treated with s.c. Sandostatin, in some instances, the state of persistent hyperglycaemia may be induced as a result of chronic administration. Hypoglycaemia has also been reported.
In patients with concomitant Type I diabetes mellitus, Sandostatin LAR is likely to affect glucose regulation, and insulin requirements may be reduced. In non-diabetics and type II diabetics with partially intact insulin reserves, Sandostatin s.c. administration may result in increases in post-prandial glycaemia. It is therefore recommended to monitor glucose tolerance and antidiabetic treatment.
In patients with insulinomas, octreotide, because of its greater relative potency in inhibiting the secretion of GH and glucagon than that of insulin, and because of the shorter duration of its inhibitory action on insulin, may increase the depth and prolong the duration of hypoglycaemia. These patients should be closely monitored.
Nutrition
Octreotide may alter absorption of dietary fats in some patients.
Depressed vitamin B12 levels and abnormal Schilling’s tests have been observed in some patients receiving octreotide therapy. Monitoring of vitamin B12 levels is recommended during therapy with Sandostatin LAR in patients who have a history of vitamin B12 deprivation.
Sodium content
Sandostatin LAR contains less than 1 mmol (23 mg) sodium per dose, i.e is essentially “sodium-free”.
4.5 Interaction with other medicinal products and other forms of interaction
None known.
4.6 Fertility, pregnancy and lactation
None known.
4.7 Effects on ability to drive and use machines
None known.
4.8 Undesirable effects
None known.
4.9. Overdose
None known.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Not applicable.
5.2
Pharmacokinetic properties
Not applicable.
5.3 Preclinical safety data
Each of the constituents are well established pharmacopoeial ingredients, so no further information is presented.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Powder (Vial):
Poly (DL-lactide-co-glycolide)
Mannitol (E421)
Solvent (Prefilled syringe):
Carmellose sodium Mannitol (E421)
Poloxamer 188 Water for injections
6.2 Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products.
6.3 Shelf life
3 years
The product must not be stored after reconstitution (must be used immediately).
6.4 Special precautions for storage
Store in the original package in order to protect from light.
Store in a refrigerator (2°C to 8°C). Do not freeze.
Sandostatin LAR may be stored below 25°C on the day of the injection.
6.5 Nature and contents of container
Unit packs containing one 6 mL glass vial with rubber stopper (bromobutyl rubber), sealed with an aluminium flip-off seal, containing powder for suspension for injection and one 3 mL colourless pre-filled glass syringe with front and plunger stopper
(chlorobutyl rubber) with 2 mL solvent, co-packaged in a sealed blister tray with one vial adapter and one safety injection needle.
Multipacks of three unit packs, each unit pack containing: one 6 mL glass vial with rubber stopper (bromobutyl rubber), sealed with an aluminium flip-off seal, containing powder for suspension for injection and one 3 mL colourless pre-filled glass syringe with front and plunger stopper (chlorobutyl rubber) with 2 mL solvent, co-packaged in a sealed blister tray with one vial adapter and one safety injection needle.
Not all pack sizes may be marketed
6.6 Special precautions for disposal and other handling
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
Instructions for preparation and intramuscular injection for Sandostatin LAR
FOR DEEP INTRAMUSCULAR INJECTION ONLY
Included in the injection kit:
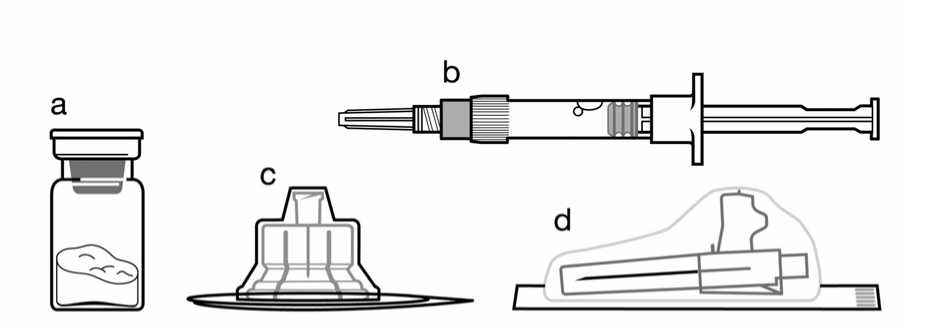
a. One vial containing Sandostatin LAR powder,
b. One prefilled syringe containing the vehicle solution for reconstitution,
c. One vial adapter for drug product reconstitution,
d. One safety injection needle.
Follow the instructions below carefully to ensure proper reconstitution of Sandostatin LAR before deep intramuscular injection.
There are 3 critical actions in the reconstitution of Sandostatin LAR. Not following them could result in failure to deliver the drug appropriately.
• The injection kit must reach room temperature. Remove the injection kit from the fridge and let the kit stand at room temperature for a minimum of 30 minutes before reconstitution, but do not exceed 24 hours.
• After adding the diluent solution, ensure that the powder is fully saturated by letting the vial stand for 5 minutes.
• After saturation, shake the vial moderately in a horizontal direction for a minimum of 30 seconds until a uniform suspension is formed. The Sandostatin LAR suspension must only be prepared immediately before administration.
Sandostatin LAR should only be administered by a trained healthcare professional.
Step 1
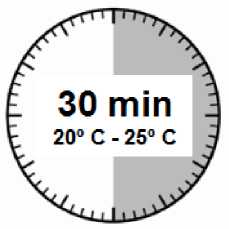
• Remove the Sandostatin LAR injection kit from refrigerated storage.
ATTENTION: It is essential to start the reconstitution process only after the injection kit reaches room temperature. Let the kit stand at room temperature for a minimum of 30 minutes before reconstitution, but do not exceed 24 hours.
Note: The injection kit can be re-refrigerated if needed.
Step 2
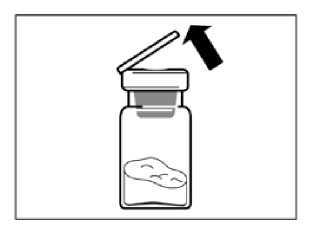
Remove the plastic cap from the vial and clean the rubber stopper of the vial with an alcohol wipe.
Remove the lid film of the vial adapter packaging, but do NOT remove the vial adapter from its packaging.
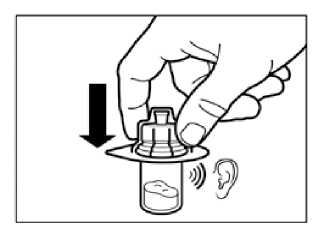
Holding the vial adapter packaging, position the vial adapter on top of the vial and push it fully down so that it snaps in place, confirmed by an audible “click.”
Lift the packaging off the vial adapter with a vertical movement.
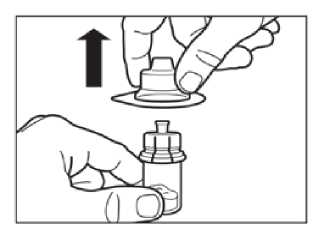
Step 3
• Remove the cap from the syringe prefilled with diluent solution and screw the syringe onto the vial adapter.
• Slowly push the plunger all the way down to transfer all the diluent solution in the vial.
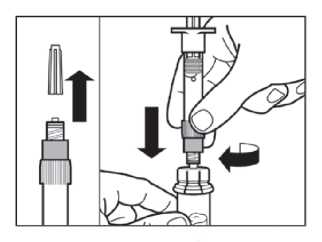
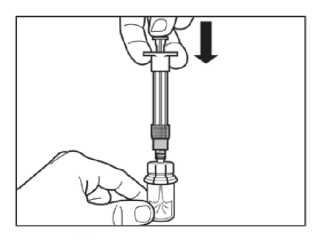
Step 4
ATTENTION: It is essential to let the vial stand for 5 minutes to ensure that the diluent has fully saturated the powder.
Note: It is normal if the plunger rod moves up as there might be a slight overpressure in the vial.
• At this stage prepare the patient for injection.
Step 5
• After the saturation period, make sure that the plunger is pushed all the way down in the syringe.
ATTENTION: Keep the plunger pressed and shake the vial moderately in a horizontal direction for a minimum of 30 seconds so that the powder is completely suspended (milky uniform suspension). Repeat moderate shaking for another 30 seconds if the powder is not completely suspended.
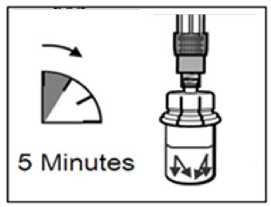
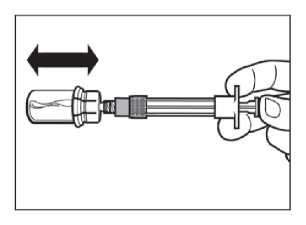
Step 6
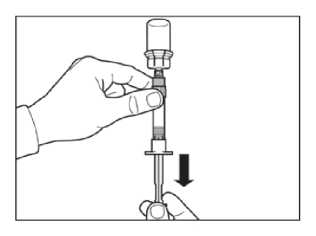
Turn syringe and vial upside down, slowly pull the plunger back and draw the entire contents from the vial into the syringe.
Unscrew the syringe from the vial adapter.
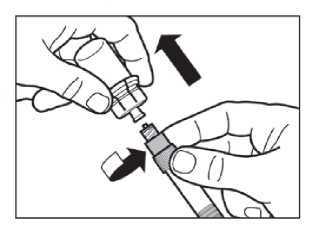
Step 7
Screw the safety injection needle onto the syringe.
If immediate administration is delayed, gently re-shake the syringe to ensure a milky uniform suspension
Prepare injection site with an alcohol wipe.
Pull the protective cover straight off the needle.
Gently tap the syringe to remove any visible bubbles expel them from the syringe.
Proceed immediately to Step 8 for administration to patient. Any delay may result in sedimentation.
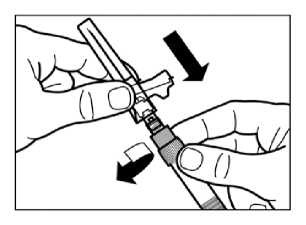
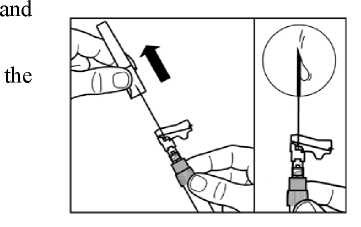
Step 8
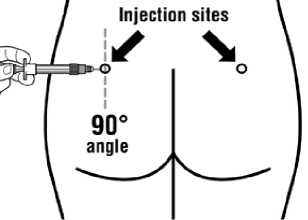
Sandostatin LAR must be given only by deep intramuscular injection, NEVER intravenously.
Insert the needle fully into the left or right gluteus at a 90° angle to the skin.
Slowly pull back the plunger to check that no blood vessel has been penetrated (reposition if a blood vessel has been penetrated).
Depress the plunger with steady pressure until the syringe is empty. Withdraw the needle from the injection site and activate the safety guard (as shown in Step 9).
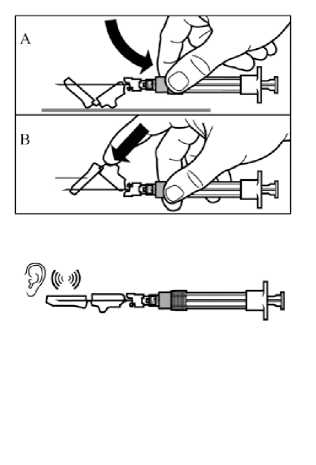
Step 9
Activate the safety guard over the needle in one of the two methods shown:
- either press the hinged section of the safety guard down onto a hard surface (figure A)
- or push the hinge forward with your finger (figure B). An audible “click” confirms the proper activation.
Dispose of syringe immediately (in a sharps container).
Administrative Data
7. Marketing Authorisation Holder
Novartis Pharmaceuticals UK Limited
(Trading as Sandoz Pharmaceuticals)
Frimley Business Park
Frimley
Camberley
Surrey
GU16 5SG
United Kingdom
8. Marketing Authorisation Number
PL 00101/0514.
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
07/06/2007
10 DATE OF REVISION OF THE TEXT
03/03/2016