Diovan 3 Mg/Ml Oral Solution
#
Package leaflet: Information for the user
Diovan 3 mg/ml oral solution
Valsartan
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Diovan is and what it is used for
2. What you need to know before you take Diovan
3. How to take Diovan
4. Possible side effects
5. How to store Diovan
6. Contents of the pack and other information Instructions for using the oral syringe and the dosing cup
1. What Diovan is and what it is used for
way
(9)

3l
(193)
Diovan contains the active substance: valsartan and belongs to a class of medicines known as angiotensin II receptor antagonists, which help to control high blood pressure. Angiotensin II is a substance in the body that causes vessels to tighten, thus causing your blood pressure to increase. Diovan works by blocking the effect of angiotensin II. As a result, blood vessels relax and blood pressure is lowered.
Diovan 3 mg/ml oral solution can be used to treat high blood pressure in children and adolescents 6 to 18 years of age.
High blood pressure increases the workload on the heart and arteries. If not treated it can damage the blood vessels of the brain, heart, and kidneys, and may result in a stroke, heart failure, or kidney failure. High blood pressure increases the risk of heart attacks. Lowering your blood pressure to normal reduces the risk of developing these disorders.
2. What you need to know before you take Diovan Do not take Diovan:
• if you are allergic (hypersensitive) to valsartan or any of the other ingredients of this medicine (listed in section 6).
• if you have severe liver disease.
• if you are more than 3 months pregnant (it is also better to avoid Diovan in early pregnancy - see pregnancy section).
• if you have diabetes or impaired kidney function and you are treated with a blood pressure lowering medicine containing aliskiren.
If any of the above apply to you, tell your doctor and do not take Diovan Warnings and precautions:
Talk to your doctor
• if you have liver disease.
• if you have severe kidney disease or if you are undergoing dialysis.
• if you are suffering from a narrowing of the kidney artery.
• if you have recently undergone kidney transplantation (received a new kidney).
• If you have ever experienced swelling of the tongue and face caused by an allergic reaction called angioedema when taking another drug (including ACE inhibitors), tell your doctor. If these symptoms occur when you are taking Diovan, stop taking Diovan immediately and never take it again. See also section 4, “Possible side effects".
• if you are taking medicines that increase the amount of potassium in your blood. These include potassium supplements or salt substitutes containing potassium, potassium-sparing medicines and heparin. It may be necessary to check the amount of potassium in your blood at regular intervals.
• if you suffer from aldosteronism. This is a disease in which your adrenal glands make too much of the hormone aldosterone. If this applies to you, the use of Diovan is not recommended.
• if you have lost a lot of fluid (dehydration) caused by diarrhoea, vomiting, or high doses of water tablets (diuretics).
• if you are taking any of the following medicines used to treat high blood pressure:
° an ACE inhibitors (for example enalapril, lisinopril, ramipril), in particular if you have diabetes-related kidney problems.
° aliskiren
Your doctor may check your kidney function, blood pressure, and the amount of electrolytes (e.g. potassium) in your blood at regular intervals.
See also information under the heading “Do not take Diovan"
You must tell your doctor if you think you are (or might become) pregnant. Diovan is not recommended in early pregnancy, and must not be taken if you are more than 3 months pregnant, as it may cause serious harm to your baby if used at that stage (see pregnancy section).
Other medicines and Diovan
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
The effect of the treatment can be influenced if Diovan is taken together with certain other medicines. It may be necessary to change the dose, to take other precautions, or in some cases to stop taking one of the medicines. This applies to both prescription and non-prescription medicines, especially:
• other medicines that lower blood pressure, especially water tablets (diuretics), ACE inhibitors (such as enalapril, lisinopril, etc.,) or aliskiren (see also information under the headings “Do not take Diovan" and “Warnings and precautions").
• medicines that increase the amount of potassium in your blood. These include potassium supplements or salt substitutes containing potassium, potassium-sparing medicines and heparin.
• certain type of pain killers called non-steroidal anti-inflammatory medicines (NSAIDs).
• some antibiotics (rifamycin group), a drug used to protect against transplant rejection (ciclosporin) or an antiretroviral drug used to treat HIV/AIDS infection (ritonavir). These drugs may increase the effect of Diovan.
• lithium, a medicine used to treat some types of psychiatric illness.
Pregnancy and breast-feeding
• You must tell your doctor if you think that you are (or might become) pregnant. Your doctor will normally advise you to stop taking Diovan before you become pregnant or as soon as you know you are pregnant, and will advise you to take another medicine instead of Diovan. Diovan is not recommended in early pregnancy, and must not be taken when more than 3 months pregnant, as it may cause serious harm to your baby if it is used after the third month of pregnancy.
• Tell your doctor if you are breast-feeding or about to start breast-feeding. Diovan is not recommended for mothers who are breast-feeding, and your doctor may choose another treatment for you if you wish to breast-feed, especially if your baby is newborn, or was born prematurely.
Driving and using machines
Before you drive a vehicle, use tools or operate machines, or carry out other activities that require concentration, make sure you know how Diovan affects you. Like many other medicines used to treat high blood pressure, Diovan may in rare cases cause dizziness and affect the ability to concentrate.
Diovan contains sucrose, methylparahydroxybenzoate and poloxamer
• Diovan solution contains 0.3 g sucrose per millilitre. Take this into account if you have diabetes mellitus. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking Diovan solution. The amount of sucrose in the Diovan solution may be harmful to your teeth.
• Diovan solution contains methyl parahydroxybenzoate (E218). This may cause allergic reactions possibly occuring some time after you take the solution. The signs may include rash, itching, hives. If any of the side effects gets serious. please tell your doctor.
• Diovan solution contains poloxamer (188). This may soften your stools.
3. How to take Diovan
Always take Diovan exactly as your doctor has told you in order to get the best results and reduce the risk of side effects. You should check with your doctor or pharmacist if you are not sure. People with high blood pressure often do not notice any signs of this problem. Many may feel quite normal. This makes it all the more important for you to keep your appointments with the doctor even if you are feeling well.
Please read the instructions at the end of this leaflet before you use the oral syringe or the dosing cup.
How much to take
Diovan solution should be taken once a day
• If you weigh less than 35 kg
° the usual dose is 20 mg of valsartan (corresponding to 7 ml of the solution).
• If you weigh 35 kg or more:
° the usual dose is 40 mg of valsartan (corresponding to 13 ml of the solution).
In some cases your doctor may ask you to take
• up to 40 mg of valsartan (corresponding to 13 ml of the solution) for those weighing less than 35 kg.
• up to 80 mg valsartan (corresponding to 27 ml of the solution) for those weighing 35 kg or more.
You can take Diovan with or without food.
Take Diovan at about the same time each day.
If you take more Diovan than you should
If you experience severe dizziness and/or fainting, contact your doctor immediately and lie down. If you have accidentally taken more Diovan solution than you should, contact your doctor, pharmacist, or hospital.
If you forget to take Diovan
If you forget to take a dose, take it as soon as you remember. However, if it is almost time for your next dose, skip the dose you missed.
Do not take a double dose to make up for a forgotten dose.
If you stop taking Diovan
Stopping your treatment with Diovan may cause your disease to get worse. Do not stop taking your medicine unless your doctor tells you to.
If you have further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Some side effects can be serious and need immediate medical attention:
You may experience symptoms of angioedema (a specific allergic reaction), such as
• swollen face, lips, tongue or throat
• difficulty in breathing or swallowing
• hives, itching
If you get any of these symptoms, stop taking Diovan and contact your doctor straight away (see also section 2 “Warnings and precautions”).
Side effects include:
Common (may affect up to 1 in 10 people):
• dizziness
• low blood pressure with or without symptoms such as dizziness and fainting when standing up
• decreased kidney function (signs of renal impairment)
Uncommon (may affect up to 1 in 100 people):
• angioedema (see section “Some symptoms need immediate medical attention")
• sudden loss of consciousness (syncope)
• spinning sensation (vertigo)
• severely decreased kidney function (signs of acute renal failure)
• muscle spasms, abnormal heart rhythm (signs of hyperkalaemia)
• breathlessness, difficulty breathing when lying down, swelling of the feet or legs (signs of cardiac failure)
• headache
• cough
• abdominal pain
• nausea
• diarrhoea
• tiredness
• weakness
Not known (frequency cannot be estimated from the available data):
• blistering skin (sign of dermatitis bullous)
• allergic reactions with rash, itching and hives; symptoms of fever, swollen joints and joint pain, muscle pain, swollen lymph nodes and/or flu-like symptoms may occur (signs of serum sickness)
• purplish-red spots, fever, itching (signs of inflammation of blood vessels also called vasculitis)
• unusual bleeding or bruising (signs of thrombocytopenia)
• muscle pain (myalgia)
• fever, sore throat or mouth ulcers due to infections (symptoms of low level of white blood cells also called neutropenia)
5032577 R91
29.05.15 15:28
5032577_R91.indd 1
• decrease of level of haemoglobin and decrease of the percentage of red blood cells in the blood (which can lead to anaemia in severe cases)
• increase of level of potassium in the blood (which can trigger muscle spasms and abnormal heart rhythm in severe cases)
• elevation of liver function values (which can indicate liver damage) including an increase of bilirubin in the blood (which can trigger yellow skin and eyes in severe cases)
• increase of level of blood urea nitrogen and increase of level of serum creatinine (which can indicate abnormal kidney function)
The frequency of some side effects may vary depending on your condition. For example, side effects such as dizziness, and decreased kidney function, were seen less frequently in adult patients treated with high blood pressure than in adult patients treated for heart failure or after a recent heart attack.
Side effects in children and adolescents are similar to those seen in adults.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly (see details below). By reporting side effects you can help provide more information on the safety of this medicine.
United Kingdom:
Yellow Card Scheme
Website: www.mhra.gov.uk/yellowcard
Ireland:
HPRA Pharmacovigilance, Earlsfort Terrace, IRL - Dublin 2; Tel: +353 1 6764971; Fax: +353 1 6762517. Website: www.hpra.ie; E-mail: medsafety@hpra.ie.
Malta:
ADR Reporting
Website: www.medicinesauthority.gov.mt/adrportal
5. How to store Diovan
• Keep this medicine out of the sight and reach of children.
• Do not use this medicine after the expiry date which is stated on the bottle. The expiry date refers to the last day of that month.
• Do not store above 30°C. Once opened, the bottle can be stored for up to 3 months at temperatures below 30°C.
• Do not use this medicine if you notice that the pack is damaged or shows signs of tampering.
• Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information What Diovan contains
• The active substance is valsartan. Each ml of oral solution contains 3 mg of valsartan.
• The other ingredients are sucrose, methyl parahydroxybenzoate (E218), potassium sorbate, poloxamer (188), citric acid anhydrous, sodium citrate, artificial blueberry flavour, propylene glycol (E1520), sodium hydroxide, hydrochloric acid, purified water.
What Diovan looks like and contents of the pack
Diovan 3 mg/ml oral solution is a clear, colourless to pale yellow solution.
• The solution is supplied in a pack containing one 180 ml amber glass bottle with a child-resistant screw cap and a yellow tamper evident ring. The bottle contains 160 ml of solution. It comes with a dispensing kit containing one press in bottle adapter, one 5 ml oral polypropylene dosing syringe and one 30 ml polypropylene dosing cup.
Marketing Authorisation Holder and Manufacturer
The product licence holder is:
Novartis Pharmaceuticals UK Limited,
Frimley Business Park,
Frimley, Camberley,
Surrey GU16 7SR,
United Kingdom
Diovan Solution is released onto the market by:
Novartis Pharma GmBH Roonstrasse 25,
D-90429 Nurnberg,
Germany
If you would like any more information, or would like this leaflet in a different format, please contact Medical Information at Novartis Pharmaceuticals UK Ltd., telephone number 01276 698370, or please contact Novartis Ireland Limited, Beech House, Beech Hill Office Campus, Clonskeagh, Dublin 4.
This leaflet was approved in April 2015.
This medicinal product is authorised in the Member States of the EEA under the following names:
|
Austria, Bulgaria, Cyprus, Czech Republic, Denmark, Estonia, Finland, Germany, Greece, Hungary, Iceland, Ireland, Latvia, Lithuania, Malta, Netherlands, Norway, Poland, Portugal, Romania, Slovak Republic, Slovenia, Spain, Sweden, United Kingdom |
Diovan |
|
Belgium, Luxembourg |
Diovane |
|
France, Italy |
Tareg |
INSTRUCTIONS FOR USING THE ORAL SYRINGE AND THE DOSING CUP
Please read these instructions carefully before taking the medicine. This will help you to use the oral syringe and the dosing cup correctly.
What you will be using


A press in bottle adapter:
• that you insert into the neck of the bottle.
• Once you have inserted it, do not remove it.
A bottle containing the medicine:
• that has a child- resistant screw cap.
• Always screw the cap back on after use.
An oral dosing syringe:
• that consists of a clear plastic tube with a plunger inside.
• The oral syringe fits into the bottle adapter and is used to measure out the required amount of medicine from the bottle. Use a new bottle adapter and oral dosing syringe each time you start a new bottle of medicine.
A dosing cup:
• that can be used if the prescribed dose requires filling the syringe several times.
• Always put the dosing cup back onto the cap after use and cleaning.
Fitting the press in bottle adapter into a new bottle of medicine
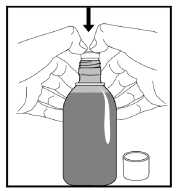
1. Remove the cap from the bottle by pushing it down firmly and turning it anti-clockwise (as shown on the top of the cap).
2. Holding the open bottle upright on a table, push the bottle adapter firmly into the neck of the bottle as far as it will go.
Note: You may not be able to push the bottle adapter down fully but this does not matter as it will be forced into the bottle when you screw the cap back on.
3. Screw the cap back on the bottle.
Preparing a dose of medicine

4. Remove the cap from the bottle by pushing it down firmly and turning it anti-clockwise (as shown on the top of the cap).
5. Check that the plunger is pushed fully into the oral syringe.
6. Keeping the bottle upright, insert the oral syringe firmly into the bottle adapter.
7. Holding the oral syringe in place, carefully turn the bottle and oral syringe upside down.
8. Before you measure your dose you need to get rid of any large bubbles that may be trapped in the oral syringe. To do this:
• slowly pull the plunger all the way out so that the oral syringe fills with medicine.
• then, push the plunger all the way back in so that it is empty again.
Measuring a dose of medicine
Note: The total amount of solution that can be measured into the oral syringe is 5 ml. Depending on the prescribed dose, it may be necessary to repeat steps 10 to 16 several times. For example, if the prescribed dose is 13 ml, it will be necessary to measure out the solution in three separate stages: 5 ml + 5 ml + 3 ml.
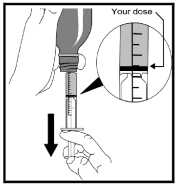
9. Find the marker on the oral syringe that corresponds to the required amount of medicine.
10. Slowly pull the plunger out until the top edge of the black ring inside is exactly level with the marker.
11. Carefully turn the bottle and oral syringe back upright.
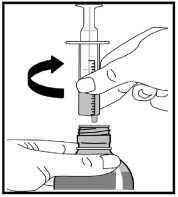
12. Remove the oral syringe from the bottle adapter by gently twisting it out.
Taking the medicine
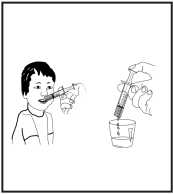
13. Sit upright.
14. Put the end of the oral syringe into the mouth.
15. Slowly push the plunger in and swallow the medicine directly from the oral syringe.
16. If the prescribed dose requires filling the syringe several times, you can empty the measured doses of medicine from the syringe into the dosing cup and then check the total volume of solution.
17. Drink the entire solution right away.
18. Replace the child-resistant screw cap after use.
19. To clean the oral syringe:
• Wipe the outside of the oral syringe with a clean, dry tissue.
• Do this after each time that you use the oral syringe.
20. To clean the cup:
• Rinse the cup with clean water.
• Dry the cup with a clean tissue and place it back over the cap of the bottle.
5032577 R91
5032577_R91.indd 2
29.05.15 15:28