Docetaxel Mylan 40 Mg/Ml Concentrate And Solvent For Solution For Infusion
PACKAGE LEAFLET: INFORMATION FOR THE USER Docetaxel 40 mg/ml concentrate and solvent for solution for infusion
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or your hospital pharmacist.
- If any of the side effects gets serious or if you notice any side effects not listed in this leaflet, please tell your doctor or hospital pharmacist.
In this leaflet:
1. What Docetaxel 40 mg/ml concentrate and solvent for solution for infusion is and what it is used for
2. Before you use Docetaxel 40 mg/ml concentrate and solvent for solution for infusion
3. How to use Docetaxel 40 mg/ml concentrate and solvent for solution for infusion
4. Possible side-effects
5. How to store Docetaxel 40 mg/ml concentrate and solvent for solution for infusion
6. Further information
1. WHAT DOCETAXEL 40 MG/ML CONCENTRATE AND SOLVENT FOR SOLUTION FOR INFUSION IS AND WHAT IT IS USED FOR
The name of this medicine is Docetaxel 40 mg/ml concentrate and solvent for solution for infusion. Docetaxel is a substance derived from the needles of yew trees.
Docetaxel belongs to the group of anti-cancer medicines called taxoids.
Docetaxel 40 mg/ml concentrate and solvent for solution for infusion has been prescribed by your doctor for the treatment of breast cancer, special forms of lung cancer (non-small cell lung cancer), prostate cancer, gastric cancer or head and neck cancer:
- For the treatment of advanced breast cancer, Docetaxel 40 mg/ml concentrate and solvent for solution for infusion could be administered either alone or in combination with doxorubicin, or trastuzumab, or capecitabine.
- For the treatment of early breast cancer with or without lymph node involvement, Docetaxel 40 mg/ml concentrate and solvent for solution for infusion could be administered in combination with doxorubicin and cyclophosphamide.
- For the treatment of lung cancer, Docetaxel 40 mg/ml concentrate and solvent for solution for infusion could be administered either alone or in combination with cisplatin.
- For the treatment of prostate cancer, Docetaxel 40 mg/ml concentrate and solvent for solution for infusion is administered in combination with prednisone or prednisolone.
- For the treatment of metastatic gastric cancer, Docetaxel 40 mg/ml concentrate and solvent for solution for infusion is administered in combination with cisplatin and 5-fluorouracil.
- For the treatment of head and neck cancer, Docetaxel 40 mg/ml concentrate and solvent for solution for infusion is administered in combination with cisplatin and 5-fluorouracil.
2. BEFORE YOU USE DOCETAXEL 40 MG/ML CONCENTRATE AND SOLVENT FOR SOLUTION FOR INFUSION
You must not be given Docetaxel 40 mg/ml concentrate and solvent for solution for infusion if
• you are allergic (hypersensitive) to docetaxel or any of the other ingredients of Docetaxel 40 mg/ml concentrate and solvent for solution for infusion.
• the number of white blood cells is too low.
• you have a severe liver disease.
Take special care with Docetaxel 40 mg/ml concentrate and solvent for solution for infusion
Before each treatment with Docetaxel 40 mg/ml concentrate and solvent for solution for infusion, you will have blood tests to check that you have enough blood cells and sufficient liver function to receive Docetaxel 40 mg/ml concentrate and solvent for solution for infusion. In case of white blood cells disturbances, you may experience associated fever or infections.
You will be asked to take premedication consisting of an oral corticosteroid such as dexamethasone, one day prior to Docetaxel 40 mg/ml concentrate and solvent for solution for infusion administration and to continue for one or two days after it in order to minimise certain undesirable effects which may occur after the infusion of Docetaxel 40 mg/ml concentrate and solvent for solution for infusion in particular allergic reactions and fluid retention (swelling of the hands, feet, legs or weight gain).
During treatment, you may be given medication to maintain the number of your blood cells.
The 1.8 ml solvent vial contains small amounts of ethanol, less than 100mg per dose.
The 7.1 ml solvent vial contains 13 vol % ethanol (alcohol), i.e. up to 152.1 mg per dose, equivalent to 3 ml beer, 1.3 ml wine per dose.
Harmful for those suffering from alcoholism.
To be taken into account in pregnant or breast-feeding women, children and high-risk groups such as patients with liver disease or epilepsy.
Taking other medicines
Please tell your doctor or hospital pharmacist if you are taking or have recently taken any other medicine, including medicines obtained without a prescription. This is because Docetaxel 40 mg/ml concentrate and solvent for solution for infusion or the other medicine may not work as well as expected and you may be more likely to get a side effect.
Pregnancy
Ask your doctor for advice before being given any medicine.
Docetaxel 40 mg/ml concentrate and solvent for solution for infusion must NOT be administered if you are pregnant unless clearly indicated by your doctor.
You must not become pregnant during treatment with this medicine and must use an effective method of contraception during therapy , because Docetaxel 40 mg/ml concentrate and solvent for solution for infusion may be harmful for the unborn baby. If pregnancy occurs during your treatment, you must immediately inform your doctor.
If you are a man being treated with Docetaxel 40 mg/ml concentrate and solvent for solution for infusion you are advised not to father a child during and up to 6 months after treatment and to seek advice on conservation of sperm prior to treatment because docetaxel may alter male fertility.
Breast-feeding
You must not breast-feed while you are treated with Docetaxel 40 mg/ml concentrate and solvent for solution for infusion.
Driving and using machines
There is no reason why you cannot drive between courses of Docetaxel 40 mg/ml concentrate and solvent for solution for infusion except if you feel dizzy or are unsure of yourself.
3. HOW TO USE DOCETAXEL 40 MG/ML CONCENTRATE AND SOLVENT FOR SOLUTION FOR INFUSION
Docetaxel 40 mg/ml concentrate and solvent for solution for infusion will be administered to you by a healthcare professional.
Usual dosage
The dose will depend on your weight and your general condition. Your doctor will calculate your body surface area in square meters (m2) and will determine the dose you should receive.
Method and route of administration
Docetaxel 40 mg/ml concentrate and solvent for solution for infusion will be given by infusion into one of your veins (intravenous use). The infusion will last approximately one hour during which you will be in the hospital.
Frequency of administration
You should usually receive your infusion once every 3 weeks.
Your doctor may change the dose and frequency of dosing depending on your blood tests, your general condition and your response to Docetaxel 40 mg/ml concentrate and solvent for solution for infusion. In particular, please inform your doctor in case of diarrhoea, sores in the mouth, feeling of numbness or pins and needles, fever and give her/him results of your blood tests. Such information will allow her/him to decide whether a dose reduction is needed. If you have any further questions on the use of this product, ask your doctor, or hospital pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Docetaxel 40 mg/ml concentrate and solvent for solution for infusion can cause side effects, although not everybody gets them.
Your doctor will discuss these with you and will explain the potential risks and benefits of your treatment.
The frequency of possible side effects listed below is defined using the following convention: very common (affects more than 1 user in 10); common (affects 1 to 10 users in 100); uncommon (affects 1 to 10 users in 1,000); rare (affects 1 to 10 users in 10,000); very rare (affects less than 1 user in 10,000); not known (frequency cannot be estimated from the available data).
The most commonly reported adverse reactions of Docetaxel 40 mg/ml concentrate and solvent for solution for infusion alone are: decrease in the number of red blood cells or white blood cells, alopecia, nausea, vomiting, sores in the mouth, diarrhea and tiredness.
The severity of adverse events of Docetaxel 40 mg/ml concentrate and solvent for solution for infusion may be increased when Docetaxel 40 mg/ml concentrate and solvent for solution for infusion is given in combination with other chemotherapeutic agents.
During the infusion at the hospital the following allergic reactions (experienced in more than 1 person in 10) may occur:
• flushing, skin reactions, itching
• chest tightness; difficulty in breathing
• fever or chills
• back pain
• low blood pressure.
More severe reactions may occur.
The hospital staff will monitor your condition closely during treatment. Tell them immediately if you notice any of these effects.
Between infusions of Docetaxel 40 mg/ml concentrate and solvent for solution for infusion the following may occur, and the frequency may vary with the combinations of drugs that are received:
Very common (experienced in more than 1 in 10 patients):
• infections, decrease in the number of red (anaemia), or white blood cells (which are important in fighting infection) and platelets
• fever: if this happens you must tell your doctor immediately
• allergic reactions as described above
• loss of appetite (anorexia)
• insomnia
• feeling of numbness or pins and needles or pain in the joints of muscles
• headache
• alteration in sense of taste
• inflammation of the eye or increased tearing of the eyes
• swelling caused by faulty lymphatic drainage
• shortness of breath
• nasal drainage; inflammation of the throat and nose; cough
• bleeding from the nose
• sores in the mouth
• stomach upsets including nausea, vomiting and diarrhea, constipation
• abdominal pain
• indigestion
• short term hair loss (in most cases normal hair growth should return)
• redness and swelling of the palms of your hands or soles of your feet which may cause your skin to peel (this may also occur on the arms, face, or body)
• change in the color of your nails, which may detach
• muscle aches and pains; back pain or bone pain
• change or absence of menstrual period
• swelling of the hands, feet, legs
• tiredness; or flu-like symptoms
• weight gain or loss.
Common (experienced in less than 1 in 10 but more than 1 in 100 patients):
• oral candidiasis
• dehydration
• dizziness
• hearing impaired
• decrease in blood pressure; irregular or rapid heart beat
• heart failure
• oesophagitis
• dry mouth
• difficulty or painful swallowing
• haemorrhage
• raised liver enzymes (hence the need for regular blood tests).
Uncommon (experienced in more than 1 in 1,000 but less than 1 in 100 patients):
• fainting
• at the injection site, skin reactions, phlebitis (inflammation of the vein) or swelling
• inflammation of the colon, small intestine; intestinal perforation
• blood clots.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or hospital pharmacist.
5. HOW TO STORE DOCETAXEL 40 MG/ML CONCENTRATE AND SOLVENT FOR SOLUTION FOR INFUSION
Keep out of the reach and sight of children.
Do not use Docetaxel 40 mg/ml concentrate and solvent for solution for infusion after the expiry date which is stated on the carton and vial labels after EXP. The expiry date refers to the last day of that month.
Do not store above 25 °C or below 2 °C.
Keep the vial in the outer carton, in order to protect from light.
The premix solution should be used immediately after preparation. However the chemical and physical in-use stability of the premix solution has been demonstrated for 8 hours when stored either between 2 °C and 8 °C or at room temperature (below 25 °C).
Chemical and physical in-use stability of the infusion solution has been demonstrated for 4 hours at 2 - 8 °C and 4 hours at room temperature (below 25 °C).
From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2 to 8°C, unless reconstitution/dilution has taken place in controlled and validated aseptic conditions.
6. FURTHER INFORMATION
What the Docetaxel 40 mg/ml concentrate and solvent for solution for infusion concentrate vial contains
- The active substance is docetaxel.
Each single-dose vial of Docetaxel concentrate contains 40 mg/ml docetaxel (anhydrous).
- The other ingredients are polysorbate 80 and anhydrous citric acid.
What the solvent vial contains
13% (w/w) anhydrous ethanol in water for injections.
What Docetaxel 40 mg/ml concentrate and solvent for solution for infusion looks like and contents of the pack
Each pack contains:
• one single-dose vial of concentrate
• one single-dose vial of solvent
Two pack sizes are available:
• carton containing a 20 mg/0.5 ml concentrate for solution for infusion vial and a 1.8 ml solvent vial.
• carton containing a 80 mg/2 ml concentrate for solution for infusion vial and a
7.1 ml solvent vial.
The concentrate for solution for infusion is a clear colorless to pale yellow viscous solution.
The solvent is a colorless solution.
Not all pack sizes may be marketed
Marketing Authorisation Holder
Mylan,
Potters Bar,
Hertfordshire, EN6 1TL,
United Kingdom
Manufacturer
Agila Specialties Polska Sp. z o.o.
10, Daniszewska Str 03-230 Warsaw Poland
This leaflet was last revised in 02/2015
The following information is intended for medical or healthcare professionals only:
PREPARATION GUIDE FOR USE WITH DOCETAXEL 40 MG/ML CONCENTRATE AND SOLVENT FOR SOLUTION FOR INFUSION
It is important that you read the entire contents of this procedure prior to the preparation of either the Docetaxel 40 mg/ml concentrate premix solution or the Docetaxel 40 mg/ml concentrate infusion solution
1. FORMULATION
Docetaxel concentrate for solution for infusion is a clear colorless to pale yellow viscous solution containing 40 mg/ml docetaxel (anhydrous) in polysorbate 80. The solvent for Docetaxel 40 mg/ml is a 13% w/w solution of anhydrous ethanol in water for injections.
2. PRESENTATION
Docetaxel 40 mg/ml concentrate and solvent for solution for infusion is supplied as single-dose vials.
Two pack sizes are available:
• A box containing one concentrate vial (20 mg) and one corresponding solvent vial.
• A box containing one concentrate vial (80 mg) and one corresponding solvent vial.
Docetaxel 40 mg/ml concentrate and solvent for solution for infusion vials should be stored between 2 °C and 25 °C and protected from light.
Docetaxel 40 mg/ml concentrate and solvent for solution for infusion should not be used after the expiry date shown on the carton, blister pack and vials.
2.1 Docetaxel 40 mg/ml concentrate vials
20 mg/0.5 ml concentrate for solution for infusion vial
• 5 ml clear glass vial with a violet flip-off aluminium seal.
• Each vial contains 0.5 ml of a 40 mg/ml solution of docetaxel in polysorbate 80 (fill volume:26.8 mg/0.67 ml). This volume has been established during the development to compensate for liquid loss during preparation of the premix (see section 4) due to foaming, adhesion to the walls of the vial and "dead-volume". This overfill ensures that after dilution with the entire contents of the accompanying solvent , there is a minimal extractable premix volume of 2 ml containing 10 mg/ml docetaxel which corresponds to 20 mg per vial.
80 mg/2 ml concentrate for solution for infusion vial
• 10 ml clear glass vial with a violet flip-off aluminium seal.
• Each vial contains 2 ml of a 40 mg/ml solution of docetaxel in polysorbate 80 (fill volume:96 mg/2.4 ml). This volume has been established during the development to compensate for liquid loss during preparation of the premix (see section 4) due to foaming, adhesion to the walls of the vial and "dead-volume". This overfill ensures that after dilution with the entire contents of the accompanying solvent , there is a minimal extractable premix volume of 8 ml containing 10 mg/ml docetaxel which corresponds to 80 mg per vial.
2.2 Solvent for Docetaxel 40mg/ml
The solvent is a 13% w/w solution of anhydrous ethanol in water for injections.
1.8 ml solvent vial
• The solvent vial is a 5 ml clear glass vial with a green flip-off aluminium seal.
• Each solvent vial contains 1.8 ml (fill volume 2.0 ml). This volume has been established based on the fill volume of the concentrate vial. The addition of the entire contents of the solvent vial to the contents of the Docetaxel 40 mg/ml concentrate vial ensures a premix concentration of 10 mg/ml docetaxel.
7.1 ml solvent vial
• The solvent for Docetaxel 40 mg/ml is a 10 ml clear glass vial with a green flip-off aluminium seal.
• Each solvent vial contains 7.1 ml (fill volume 7.5 ml). This volume has been established based on the fill volume of the concentrate vial. The addition of the entire contents of the solvent vial to the contents of the Docetaxel 40 mg/ml concentrate vial ensures a premix concentration of 10 mg/ml docetaxel.
3. RECOMMENDATIONS FOR THE SAFE HANDLING
Docetaxel is an antineoplastic agent and, as with other potentially toxic compounds, caution should be exercised when handling it and preparing docetaxel solutions. The use of gloves is recommended.
If docetaxel concentrate, premix solution or infusion solution should come into contact with skin, wash immediately and thoroughly with soap and water. If docetaxel concentrate, premix solution or infusion solution should come into contact with mucous membranes, wash immediately and thoroughly with water.
4. PREPARATION FOR THE INTRAVENOUS ADMINISTRATION
4.1 Preparation of the docetaxel premix solution (10 mg docetaxel/ml)
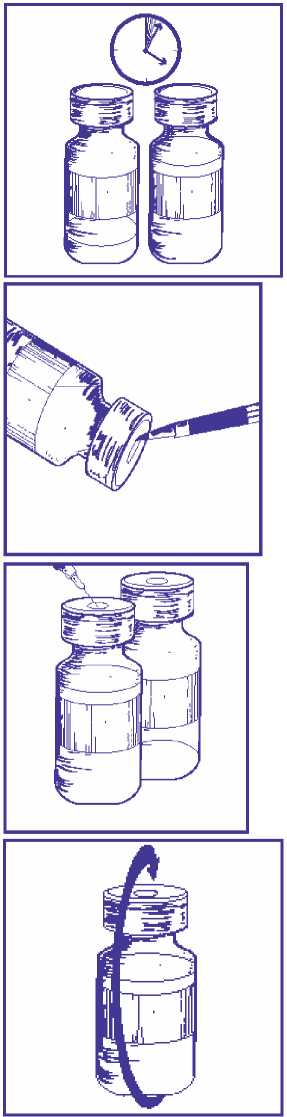
4.1.1 If the vials are stored under refrigeration, allow the required number of Docetaxel 40 mg/ml concentrate and solvent for solution for infusion boxes to stand at room temperature (below 25 °C) for 5 minutes.
4.1.2 Using a syringe fitted with a needle, aseptically withdraw the entire contents of the solvent vial by partially inverting the vial.
4.1.3 Inject the entire contents of the syringe into the corresponding Docetaxel 40 mg/ml concentrate vial.
4.1.4 Remove the syringe and needle and mix manually by repeated inversions for at least 45 seconds. Do not shake.
4.1.5 Allow the premix vial to stand for 5 minutes at room temperature (below 25 °C) and then check that the solution is homogenous and clear (foaming is normal even after 5 minutes due to the presence of polysorbate 80 in the formulation).
The premix solution contains 10 mg/ml docetaxel and should be used immediately after preparation. However the chemical and physical in-use stability of the premix solution has been demonstrated for 8 hours when stored either between + 2 °C and + 8 °C or at room temperature (below 25 °C).
4.2 Preparation of the infusion solution
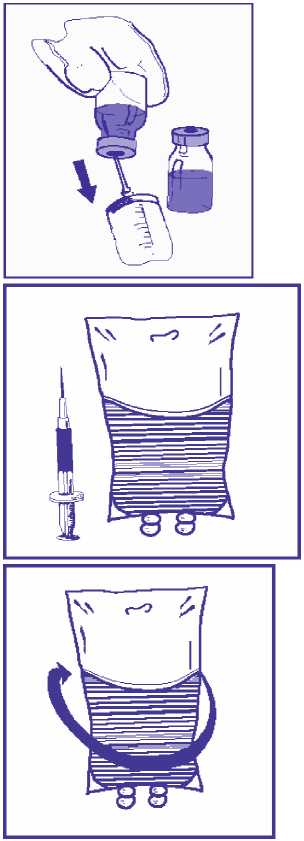
4.2.1 More than one premix vial may be necessary to obtain the required dose for the patient. Based on the required dose for the patient expressed in mg, aseptically withdraw the corresponding premix volume containing 10 mg/ml docetaxel from the appropriate number of premix vials using graduated syringes fitted with a needle. For example, a dose of 140 mg docetaxel would require 14 ml docetaxel premix solution.
4.2.2 Inject the required premix volume into a 250 ml infusion bag or bottle containing either 5% glucose solution or 0.9% sodium chloride solution. If a dose greater than 200 mg of docetaxel is required, use a larger volume of the infusion vehicle so that a concentration of 0.74 mg/ml docetaxel is not exceeded.
4.2.3 Mix the infusion bag or bottle manually using a rocking motion.
4.2.4 The docetaxel infusion solution should be used within 4 hours and should be aseptically administered as a 1-hour infusion under room temperature (below 25 °C) and normal lighting conditions.
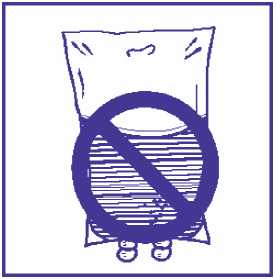
4.2.5 As with all parenteral products, the docetaxel premix solution and infusion solution should be visually inspected prior to use, solutions containing a precipitate should be discarded.
5. DISPOSAL
All materials that have been utilised for dilution and administration should be disposed of according to standard procedures.