Dorzolamide/Timolol 20 Mg/Ml + 5 Mg/Ml Preservative-Free Single Dose Eye Drops
Out of date information, search another500000/PL1a PACKAGE LEAFLET: INFORMATION FOR THE USER
COSOPT® Preservative-free 20 mg/ml + 5 mg/ml eye drops, solution, single dose container
(dorzolamide hydrochloride / timolol maleate)
The name of your medicine is COSOPT Preservative-free 20 mg/ml + 5mg/ml, eye drops, solution, single dose container. Throughout the remainder of this leaflet, it will be referred to as COSOPT Preservative-free.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What COSOPT Preservative-free is and what it is used for
2. What you need to know before you use COSOPT Preservative-free
3. How to use COSOPT Preservative-free
4. Possible side effects
5. How to store COSOPT Preservative-free
6. Contents of the pack and other information
1. What COSOPT Preservative-free is and what it is used for
COSOPT Preservative-free contains two medicines: dorzolamide and timolol.
• Dorzolamide belongs to a group of medicines called “carbonic anhydrase inhibitors”.
• Timolol belongs to a group of medicines called “beta-blockers.”
These medicines lower pressure in the eye in different ways.
COSOPT Preservative-free is prescribed to lower raised pressure in the eye in the treatment of glaucoma when beta-blocker eyedrop medicine used alone is not adequate.
2. What you need to know before you use COSOPT Preservative-free
Do not use COSOPT Preservative-free
• if you are allergic to dorzolamide hydrochloride, timolol maleate or any of the other ingredients of this medicine (listed in section 6).
• if you have now or had in the past respiratory problems, such as asthma or severe chronic obstructive bronchitis (severe lung disease which may cause wheeziness, difficulty in breathing and/or long-standing cough).
• if you have a slow heart beat, heart failure or disorders of heart rhythm (irregular heart beats).
• if you have severe kidney disease or problems, or a prior history of kidney stones.
• if you have excess acidity of the blood caused by a build up of chloride in the blood (hyperchloremic acidosis).
If you are not sure whether you should use this medicine, contact your doctor or pharmacist.
Warnings and precautions
Talk to your doctor before using COSOPT Preservative-Free
Tell your doctor about any medical or eye problems you have now or have had in the past,
• coronary heart disease (symptoms can include chest pain or tightness, breathlessness, or choking), heart failure, low blood pressure.
• disturbances of heart rate such as slow heart beat.
• breathing problems, asthma or chronic obstructive pulmonary disease.
• poor blood circulation disease (such as Raynaud's disease or Raynaud's syndrome).
• diabetes as timolol may mask signs and symptoms of low blood sugar.
• overactivity of the thyroid gland as timolol may mask signs and symptoms.
Tell your doctor before you have an operation that you are using COSOPT Preservative-free as timolol may change effects of some medicines used during anaesthesia.
Also tell your doctor about any allergies or anaphylactic reactions.
Tell your doctor if you have muscle weakness or have been diagnosed as having myasthenia gravis.
If you develop any eye irritation or any new eye problems such as redness of the eye or swelling of the eyelids, contact your doctor immediately.
If you suspect that COSOPT Preservative-free is causing an allergic reaction or hypersensitivity (for example, skin rash, severe skin reaction, or redness and itching of the eye), stop using this medicine and contact your doctor immediately.
Tell your doctor if you develop an eye infection, receive an eye injury, have eye surgery, or develop a reaction including new or worsening symptoms.
When COSOPT Preservative-free is instilled into the eye it may affect the entire body.
COSOPT Preservative-Free has not been studied in patients wearing contact lenses. If you wear soft contact lenses, you should consult your doctor before using this medicine.
Use in children
There is limited experience with COSOPT (preserved formulation) in infants and children.
Use in elderly
In studies with COSOPT (preserved formulation), the effects of COSOPT (preserved formulation) were similar in both elderly and younger patients.
Use in patients with liver impairment
Tell your doctor about any liver problems you now have or have suffered from in the past.
Other medicines and COSOPT Preservative-free
COSOPT Preservative-free can affect or be affected by other medicines you are using, including other eye drops for the treatment of glaucoma. Tell your doctor if you are using or intend to use medicines to lower blood pressure, heart medicine or medicines to treat diabetes. Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines. This is particularly important if you are;
• taking medicine to lower blood pressure or to treat heart disease (such as calcium channel blockers, beta-blockers or digoxin).
• taking medicines to treat a disturbed or irregular heartbeat such as calcium channel blockers, beta-blockers or digoxin.
• using another eye drop that contains a beta-blocker.
• taking another carbonic anhydrase inhibitor such as acetazolamide.
• taking monoamine oxidase inhibitors (MAOIs).
• taking a parasympathomimetic medicine which may have been prescribed to help you pass urine. Parasympathomimetics are also a particular type of medicine which is sometimes used to help restore normal movements through the bowel.
• taking narcotics such as morphine used to treat moderate to severe pain.
• taking medicines to treat diabetes.
• taking antidepressants known as fluoxetine and paroxetine.
• taking a sulfa medicine.
• taking quinidine (used to treat heart conditions and some types of malaria).
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before taking any medicine.
Use in pregnancy
Do not use COSOPT Preservative-free if you are pregnant unless your doctor considers it necessary.
Use in breast-feeding
Do not use COSOPT Preservative-free if you are breast-feeding. Timolol may get into your milk. Ask your doctor for advice before taking any medicine during breast-feeding.
Driving and using machines
No studies on the effects on the ability to drive or use machines have been performed. There are side effects associated with COSOPT Preservative-Free, such as blurred vision, which may affect your ability to drive and/or operate machinery. Do not drive or operate machinery until you feel well or your vision is clear.
3. How to use COSOPT Preservative-free
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure. The appropriate dosage and duration of treatment will be established by your doctor.
The recommended dose is one drop in the affected eye(s) in the morning and in the evening.
If you are using COSOPT Preservative-free with another eye drop, the drops should be instilled at least 10 minutes apart.
Do not change the dose of the medicine without consulting your doctor.
If you have difficulty administering your eye drops, seek the assistance of a family member or carer.
Do not allow the single dose container to touch the eye or areas around the eye. It could cause injury to your eye. It may also become contaminated with bacteria that can cause eye infections leading to serious damage of the eye, even loss of vision. To avoid possible contamination of the single dose container, wash your hands before using this medicine and keep the tip of the single dose container away from contact with any surface.
Instructions for use
The solution from one individual single dose container of COSOPT Preservative-free is to be used immediately after opening for administration to the affected eye(s). Since sterility cannot be maintained after the individual single dose container is opened, a new container must be opened prior to each use and must be discarded immediately after administration.
1. Open the foil sachet which contains 15 individual single dose containers. There are three strips of 5 single dose containers each in the sachet. Write the date of first opening on the sachet.
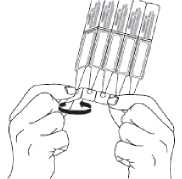
2. First wash your hands then break off one single dose container from the strip and twist open the top of the single dose container as shown.
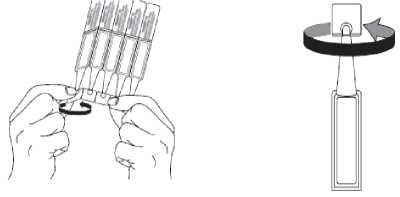
3. Tilt your head back and pull your lower eyelid down slightly to form a pocket between your eyelid and eye as shown. Do not allow any part of the container to touch your eye or any area around your eye.
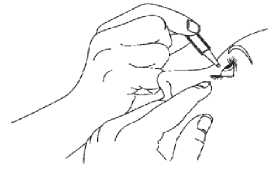
If you are not sure how to administer your medicine, ask your doctor, pharmacist or nurse.
4. Put one drop in the affected eye(s) as directed by your doctor. Do not blink while applying the drop to your eye.
Each single dose container contains enough solution for both eyes.
5. After using COSOPT Preservative-free press a finger into the corner of your eye, by the nose, or close your eyelids for 2 minutes. This helps to stop the medicine from getting into the rest of the body.
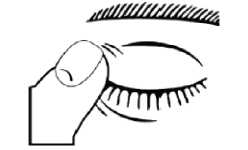
6. After putting the drop into the eye, throw away the used single dose container even if there is solution remaining to avoid contamination of the preservative free solution.
7. Store the remaining containers in the foil sachet; the remaining containers must be used within 15 days after opening of the sachet. If there are any containers left 15 days after opening the sachet they should be safely thrown away and a fresh sachet opened. It is important to continue to use the eye drops as prescribed by your doctor.
If you use more COSOPT Preservative-free than you should
If you put too many drops in your eye or swallow any of the contents of the container, among other effects, you
may become light-headed, have difficulty breathing, or feel that your heart rate has slowed. Contact your doctor
immediately.
If you forget to use COSOPT Preservative-free
It is important to take COSOPT Preservative-free as prescribed by your doctor.
If you miss a dose, use it as soon as possible. However, if it is almost time for the next dose, skip the missed dose
and go back to your regular dosing schedule.
Do not take a double dose to make up for the forgotten dose.
If you stop using COSOPT Preservative-free
If you want to stop using this medicine talk to your doctor first.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
You can usually carry on taking the drops, unless the effects are serious. If you're worried, talk to a doctor or pharmacist. Do not stop using COSOPT Preservative-free without speaking to your doctor.
Generalised allergic reactions including swelling beneath the skin can occur in areas such as the face and limbs, and can obstruct the airway which may cause difficulty swallowing or breathing, hives or itchy rash, localised and generalised rash, itchiness, severe sudden life-threatening allergic reaction.
The frequency of possible side effects listed below is defined using the following convention:
Very common (affects more than 1 user in 10)
Common (affects 1 to 10 users in 100)
Uncommon (affects 1 to 10 users in 1,000)
Rare (affects 1 to 10 users in 10,000)
Not known (frequency cannot be estimated from the available data)
The following adverse reactions have been reported with COSOPT Preservative-free or one of its components either during clinical trials or during post-marketing experience:
Very common:
Burning and stinging of the eyes, taste perversion.
Common:
Redness in and around the eye(s), watering or itching of the eye(s), corneal erosion (damage to the front layer of the eyeball), swelling and/or irritation in and around the eye(s), feeling of having something in the eye, decreased corneal sensitivity (not realizing of getting something in the eye and not feeling pain), eye pain, dry eyes, blurred vision, headache, sinusitis (feeling of tension or fullness to the nose), nausea, weakness/tiredness and fatigue.
Uncommon:
Dizziness, depression, inflammation of the iris, visual disturbances including refractive changes (due to withdrawal of miotic therapy in some cases), slow heartbeat, fainting, difficulty breathing (dyspnoea), indigestion, and kidney stones.
Rare:
Systemic lupus erythematosus (an immune disease which may cause an inflammation of internal organs), tingling or numbness of the hands or feet, insomnia, nightmares, memory loss, an increase in signs and symptoms of myasthenia gravis (muscle disorder), decreased sex drive, stroke, temporary short sightedness which may resolve when treatment is stopped, detachment of the layer below the retina that contains blood vessels following from filtration surgery which may cause visual disturbances, drooping of the eyelids (making the eye stay half closed), double vision, eyelid crusting, swelling of the cornea (with symptoms of visual disturbances), low pressure in the eye, ringing noises in your ear, low blood pressure, changes in the rhythm or speed of the heartbeat, congestive heart failure (heart disease with shortness of breath and swelling of feet and legs due to fluid build up), oedema (fluid build up), cerebral ischaemia (reduced blood supply to the brain), chest pain, palpitations (a quicker and/ or irregular heartbeat), heart attack, Raynaud's phenomenon, swelling or coldness of your hands and feet and reduced circulation in your arms and legs, leg cramps and/or leg pain when walking (claudication), shortness of breath, respiratory failure, rhinitis, nose bleed, constriction of the airways in the lungs, cough, throat irritation, dry mouth, diarrhoea, contact dermatitis, hair loss, skin rash with white silvery coloured appearance (psoriasiform rash), Peyronie's disease (which may cause a curvature of the penis), allergic type reactions such as rash, hives, itching, in rare cases possible swelling of the lips, eyes and mouth, wheezing, or severe skin reactions (Stevens Johnsons syndrome, toxic epidermal necrolysis).
Like other medicines applied into your eyes, timolol is absorbed into the blood. This may cause similar side effects as seen with oral beta-blocking agents. Incidence of side effects after topical ophthalmic administration is lower than when medicines are, for example, taken by mouth or injected. Listed additional side effects include reactions seen within the class of beta-blockers when used for treating eye conditions:
Not known:
Low blood glucose levels, heart failure, a type of heart rhythm disorder, abdominal pain, vomiting, muscle pain not caused by exercise, sexual dysfunction.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/ yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store COSOPT Preservative-free
Keep out of the sight and reach of children.
Do not use the unopened sachets of COSOPT Preservative-free after the expiry date shown by the six digits following EX (or EXP) on the foil package. The first two digits indicate the month; the last four digits indicate the year. The expiry date refers to the last day of that month.
Do not store above 30°C.
Do not freeze.
Store in the original package in order to protect from light.
You can use COSOPT Preservative-free 15 days after first opening the sachet.
Discard any unused single dose containers after this time.
Discard the opened single dose container with any remaining solution immediately after first use.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What COSOPT Preservative-free contains
- The active substances are dorzolamide hydrochloride and timolol maleate.
- Each ml contains 20 mg dorzolamide (as dorzolamide hydrochloride) and 5 mg timolol (as timolol maleate).
- The other ingredients are hydroxyethyl cellulose, mannitol, sodium citrate, sodium hydroxide and water for injections.
What COSOPT Preservative-free looks like and contents of the pack
COSOPT Preservative-Free is a clear, colourless to nearly colourless slightly viscous solution. Each aluminium sachet contains 15 low density polyethylene single dose containers containing 0.2 ml of solution.
Each pack contains 60 x 0.2 ml (4 sachets with 15 single dose containers)
Product Licence Holder
Procured from within the EU. Product Licence Holder Ginova Ltd and repackager Ginova UK Ltd both at St James' House, 8 Overcliffe, Gravesend, Kent, DA11 0HJ.
Manufacturer
Laboratoires Merck Sharp & Dohme-Chibret, Mirabel Plant - Route de Marsat, F-63963 Riom, France.
COSOPT Preservative-free 20 mg/ml + 5 mg/ml eye drops, solution, single-dose container
PL No: 18067/0458 iPOMl
This leaflet was last revised on 16th January 2015.
HOW CAN YOU OBTAIN MORE INFORMATION ABOUT COSOPT PRESERVATIVE-FREE, INCREASED EYE PRESSURE OR GLAUCOMA?
This leaflet gives the most important information about COSOPT Preservative-free. If you have any questions after you have read it, ask your doctor or pharmacist, who can give you more information
Further information about glaucoma is available from:
International Glaucoma Association (IGA),
Woodcote House, 15 Highpoint Business Village,
Henwood,
Ashford,
Kent, TN24 8DH.
Tel: 01233 648170
Registered Charity number 274681.
Alternatively, if you or someone you know has problems with their vision, and you require further advice or information, please phone the Royal National Institute for the Blind (RNIB) Helpline on 0303 123 9999, Monday to Friday 8.45am to 5.30pm, calls charged at local rate.
(The IGA and RNIB are independent UK charities and are not associated with Ginova Ltd or Ginova UK Ltd.)
COSOPT® is a registered trademark of the Merck Sharp & Dohme Corp.
Dorzolamide/Timolol 20 mg/ml + 5 mg/ml Preservative-free single dose eye drops,
(dorzolamide hydrochloride/timolol maleate)
The name of your medicine is Dorzolamide/Timolol 20 mg/ml + 5mg/ml, Preservative-free single dose eye drops. Throughout the remainder of this leaflet, it will be referred to as Dorzolamide/Timolol Preservative-free.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Dorzolamide/Timolol Preservative-free is and what it is used for
2. What you need to know before you use Dorzolamide/Timolol Preservative-free
3. How to use Dorzolamide/Timolol Preservative-free
4. Possible side effects
5. How to store Dorzolamide/Timolol Preservative-free
6. Contents of the pack and other information
1. What Dorzolamide/Timolol Preservative-free is and what it is used for
Dorzolamide/Timolol Preservative-free contains two medicines: dorzolamide and timolol.
• Dorzolamide belongs to a group of medicines called “carbonic anhydrase inhibitors”.
• Timolol belongs to a group of medicines called “beta-blockers.”
These medicines lower pressure in the eye in different ways.
Dorzolamide/Timolol Preservative-free is prescribed to lower raised pressure in the eye in the treatment of glaucoma when beta-blocker eyedrop medicine used alone is not adequate.
2. What you need to know before you use Dorzolamide/Timolol Preservative-free
Do not use Dorzolamide/Timolol Preservative-free
• if you are allergic to dorzolamide hydrochloride, timolol maleate or any of the other ingredients of this medicine (listed in section 6).
• if you have now or had in the past respiratory problems, such as asthma or severe chronic obstructive bronchitis (severe lung disease which may cause wheeziness, difficulty in breathing and/or long-standing cough).
• if you have a slow heart beat, heart failure or disorders of heart rhythm (irregular heart beats).
• if you have severe kidney disease or problems, or a prior history of kidney stones.
• if you have excess acidity of the blood caused by a build up of chloride in the blood (hyperchloremic acidosis).
If you are not sure whether you should use this medicine, contact your doctor or pharmacist.
Warnings and precautions
Talk to your doctor before using Dorzolamide/Timolol Preservative-Free
Tell your doctor about any medical or eye problems you have now or have had in the past,
• coronary heart disease (symptoms can include chest pain or tightness, breathlessness, or choking), heart failure, low blood pressure.
• disturbances of heart rate such as slow heart beat.
• breathing problems, asthma or chronic obstructive pulmonary disease.
• poor blood circulation disease (such as Raynaud's disease or Raynaud's syndrome).
• diabetes as timolol may mask signs and symptoms of low blood sugar.
• overactivity of the thyroid gland as timolol may mask signs and symptoms.
Tell your doctor before you have an operation that you are using Dorzolamide/Timolol Preservative-free as timolol may change effects of some medicines used during anaesthesia.
Also tell your doctor about any allergies or anaphylactic reactions.
Tell your doctor if you have muscle weakness or have been diagnosed as having myasthenia gravis.
If you develop any eye irritation or any new eye problems such as redness of the eye or swelling of the eyelids, contact your doctor immediately.
If you suspect that Dorzolamide/Timolol Preservative-free is causing an allergic reaction or hypersensitivity (for example, skin rash, severe skin reaction, or redness and itching of the eye), stop using this medicine and contact your doctor immediately.
Tell your doctor if you develop an eye infection, receive an eye injury, have eye surgery, or develop a reaction including new or worsening symptoms.
When Dorzolamide/Timolol Preservative-free is instilled into the eye it may affect the entire body.
Dorzolamide/Timolol Preservative-Free has not been studied in patients wearing contact lenses. If you wear soft contact lenses, you should consult your doctor before using this medicine.
Use in children
There is limited experience with Dorzolamide/Timolol (preserved formulation) in infants and children.
Use in elderly
In studies with Dorzolamide/Timolol (preserved formulation), the effects of Dorzolamide/Timolol (preserved formulation) were similar in both elderly and younger patients.
Use in patients with liver impairment
Tell your doctor about any liver problems you now have or have suffered from in the past.
Other medicines and Dorzolamide/Timolol Preservative-free
Dorzolamide/Timolol Preservative-free can affect or be affected by other medicines you are using, including other eye drops for the treatment of glaucoma. Tell your doctor if you are using or intend to use medicines to lower blood pressure, heart medicine or medicines to treat diabetes. Tell your doctor or pharmacist if you are using, have recently used or might use any other medicines. This is particularly important if you are;
• taking medicine to lower blood pressure or to treat heart disease (such as calcium channel blockers, beta-blockers or digoxin).
• taking medicines to treat a disturbed or irregular heartbeat such as calcium channel blockers, beta-blockers or digoxin.
• using another eye drop that contains a beta-blocker.
• taking another carbonic anhydrase inhibitor such as acetazolamide.
• taking monoamine oxidase inhibitors (MAOIs).
• taking a parasympathomimetic medicine which may have been prescribed to help you pass urine. Parasympathomimetics are also a particular type of medicine which is sometimes used to help restore normal movements through the bowel.
• taking narcotics such as morphine used to treat moderate to severe pain.
• taking medicines to treat diabetes.
• taking antidepressants known as fluoxetine and paroxetine.
• taking a sulfa medicine.
• taking quinidine (used to treat heart conditions and some types of malaria).
Pregnancy and breast-feeding
Ask your doctor or pharmacist for advice before taking any medicine.
Use in pregnancy
Do not use Dorzolamide/Timolol Preservative-free if you are pregnant unless your doctor considers it necessary.
Use in breast-feeding
Do not use Dorzolamide/Timolol Preservative-free if you are breast-feeding. Timolol may get into your milk. Ask your doctor for advice before taking any medicine during breast-feeding.
Driving and using machines
No studies on the effects on the ability to drive or use machines have been performed. There are side effects associated with Dorzolamide/Timolol Preservative-Free, such as blurred vision, which may affect your ability to drive and/or operate machinery. Do not drive or operate machinery until you feel well or your vision is clear.
3. How to use Dorzolamide/Timolol Preservative-free
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure. The appropriate dosage and duration of treatment will be established by your doctor.
The recommended dose is one drop in the affected eye(s) in the morning and in the evening.
If you are using Dorzolamide/Timolol Preservative-free with another eye drop, the drops should be instilled at least 10 minutes apart.
Do not change the dose of the medicine without consulting your doctor.
If you have difficulty administering your eye drops, seek the assistance of a family member or carer.
Do not allow the single dose container to touch the eye or areas around the eye. It could cause injury to your eye. It may also become contaminated with bacteria that can cause eye infections leading to serious damage of the eye, even loss of vision. To avoid possible contamination of the single dose container, wash your hands before using this medicine and keep the tip of the single dose container away from contact with any surface.
Instructions for use
The solution from one individual single dose container of Dorzolamide/Timolol Preservative-free is to be used immediately after opening for administration to the affected eye(s). Since sterility cannot be maintained after the individual single dose container is opened, a new container must be opened prior to each use and must be discarded immediately after administration.
1. Open the foil sachet which contains 15 individual single dose containers. There are three strips of 5 single dose containers each in the sachet. Write the date of first opening on the sachet.
2. First wash your hands then break off one single dose container from the strip and twist open the top of the single dose container as shown.
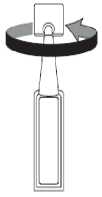
3. Tilt your head back and pull your lower eyelid down slightly to form a pocket between your eyelid and eye as shown. Do not allow any part of the container to touch your eye or any area around your eye.
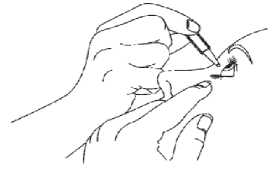
If you are not sure how to administer your medicine, ask your doctor, pharmacist or nurse.
4. Put one drop in the affected eye(s) as directed by your doctor. Do not blink while applying the drop to your eye.
Each single dose container contains enough solution for both eyes.
5. After using Dorzolamide/Timolol Preservative-free press a finger into the corner of your eye, by the nose, or close your eyelids for 2 minutes. This helps to stop the medicine from getting into the rest of the body.
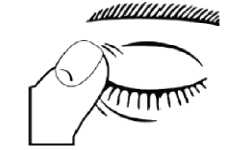
6. After putting the drop into the eye, throw away the used single dose container even if there is solution remaining to avoid contamination of the preservative free solution.
7. Store the remaining containers in the foil sachet; the remaining containers must be used within 15 days after opening of the sachet. If there are any containers left 15 days after opening the sachet they should be safely thrown away and a fresh sachet opened. It is important to continue to use the eye drops as prescribed by your doctor.
If you use more Dorzolamide/Timolol Preservative-free than you should
If you put too many drops in your eye or swallow any of the contents of the container, among other effects, you
may become light-headed, have difficulty breathing, or feel that your heart rate has slowed. Contact your doctor
immediately.
If you forget to use Dorzolamide/Timolol Preservative-free
It is important to take Dorzolamide/Timolol Preservative-free as prescribed by your doctor.
If you miss a dose, use it as soon as possible. However, if it is almost time for the next dose, skip the missed dose
and go back to your regular dosing schedule.
Do not take a double dose to make up for the forgotten dose.
If you stop using Dorzolamide/Timolol Preservative-free
If you want to stop using this medicine talk to your doctor first.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
You can usually carry on taking the drops, unless the effects are serious. If you're worried, talk to a doctor or pharmacist. Do not stop using Dorzolamide/Timolol Preservative-free without speaking to your doctor.
Generalised allergic reactions including swelling beneath the skin can occur in areas such as the face and limbs, and can obstruct the airway which may cause difficulty swallowing or breathing, hives or itchy rash, localised and generalised rash, itchiness, severe sudden life-threatening allergic reaction.
The frequency of possible side effects listed below is defined using the following convention:
Very common (affects more than 1 user in 10)
Common (affects 1 to 10 users in 100)
Uncommon (affects 1 to 10 users in 1,000)
Rare (affects 1 to 10 users in 10,000)
Not known (frequency cannot be estimated from the available data)
The following adverse reactions have been reported with Dorzolamide/Timolol Preservative-free or one of its components either during clinical trials or during post-marketing experience:
Very common:
Burning and stinging of the eyes, taste perversion.
Common:
Redness in and around the eye(s), watering or itching of the eye(s), corneal erosion (damage to the front layer of the eyeball), swelling and/or irritation in and around the eye(s), feeling of having something in the eye, decreased corneal sensitivity (not realizing of getting something in the eye and not feeling pain), eye pain, dry eyes, blurred vision, headache, sinusitis (feeling of tension or fullness to the nose), nausea, weakness/tiredness and fatigue.
Uncommon:
Dizziness, depression, inflammation of the iris, visual disturbances including refractive changes (due to withdrawal of miotic therapy in some cases), slow heartbeat, fainting, difficulty breathing (dyspnoea), indigestion, and kidney stones.
Rare:
Systemic lupus erythematosus (an immune disease which may cause an inflammation of internal organs), tingling or numbness of the hands or feet, insomnia, nightmares, memory loss, an increase in signs and symptoms of myasthenia gravis (muscle disorder), decreased sex drive, stroke, temporary short sightedness which may resolve when treatment is stopped, detachment of the layer below the retina that contains blood vessels following from filtration surgery which may cause visual disturbances, drooping of the eyelids (making the eye stay half closed), double vision, eyelid crusting, swelling of the cornea (with symptoms of visual disturbances), low pressure in the eye, ringing noises in your ear, low blood pressure, changes in the rhythm or speed of the heartbeat, congestive heart failure (heart disease with shortness of breath and swelling of feet and legs due to fluid build up), oedema (fluid build up), cerebral ischaemia (reduced blood supply to the brain), chest pain, palpitations (a quicker and/ or irregular heartbeat), heart attack, Raynaud's phenomenon, swelling or coldness of your hands and feet and reduced circulation in your arms and legs, leg cramps and/or leg pain when walking (claudication), shortness of breath, respiratory failure, rhinitis, nose bleed, constriction of the airways in the lungs, cough, throat irritation, dry mouth, diarrhoea, contact dermatitis, hair loss, skin rash with white silvery coloured appearance (psoriasiform rash), Peyronie's disease (which may cause a curvature of the penis), allergic type reactions such as rash, hives, itching, in rare cases possible swelling of the lips, eyes and mouth, wheezing, or severe skin reactions (Stevens Johnsons syndrome, toxic epidermal necrolysis).
Like other medicines applied into your eyes, timolol is absorbed into the blood. This may cause similar side effects as seen with oral beta-blocking agents. Incidence of side effects after topical ophthalmic administration is lower than when medicines are, for example, taken by mouth or injected. Listed additional side effects include reactions seen within the class of beta-blockers when used for treating eye conditions:
Not known:
Low blood glucose levels, heart failure, a type of heart rhythm disorder, abdominal pain, vomiting, muscle pain not caused by exercise, sexual dysfunction.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/ yellowcard.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Dorzolamide/Timolol Preservative-free
Keep out of the sight and reach of children.
Do not use the unopened sachets of Dorzolamide/Timolol Preservative-free after the expiry date shown by the six digits following EX (or EXP) on the foil package. The first two digits indicate the month; the last four digits indicate the year. The expiry date refers to the last day of that month.
Do not store above 30°C.
Do not freeze.
Store in the original package in order to protect from light.
You can use Dorzolamide/Timolol Preservative-free 15 days after first opening the sachet.
Discard any unused single dose containers after this time.
Discard the opened single dose container with any remaining solution immediately after first use.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Dorzolamide/Timolol Preservative-free contains
- The active substances are dorzolamide hydrochloride and timolol maleate.
- Each ml contains 20 mg dorzolamide (as dorzolamide hydrochloride) and 5 mg timolol (as timolol maleate).
- The other ingredients are hydroxyethyl cellulose, mannitol, sodium citrate, sodium hydroxide and water for injections.
What Dorzolamide/Timolol Preservative-free looks like and contents of the pack
Dorzolamide/Timolol Preservative-Free is a clear, colourless to nearly colourless slightly viscous solution. Each aluminium sachet contains 15 low density polyethylene single dose containers containing 0.2 ml of solution.
Each pack contains 60 x 0.2 ml (4 sachets with 15 single dose containers)
Product Licence Holder
Procured from within the EU. Product Licence Holder Ginova Ltd and repackager Ginova UK Ltd both at St James' House, 8 Overcliffe, Gravesend, Kent, DA11 0HJ.
Manufacturer
Laboratoires Merck Sharp & Dohme-Chibret, Mirabel Plant - Route de Marsat, F-63963 Riom, France.
Dorzolamide/Timolol 20 mg/ml + 5 mg/ml Preservative-free single-dose eye drops
PL No: 18067/0458 iPOMl
This leaflet was last revised on 16th January 2015.
HOW CAN YOU OBTAIN MORE INFORMATION ABOUT COSOPT PRESERVATIVE-FREE, INCREASED EYE PRESSURE OR GLAUCOMA?
This leaflet gives the most important information about Dorzolamide/Timolol Preservative-free. If you have any questions after you have read it, ask your doctor or pharmacist, who can give you more information
Further information about glaucoma is available from:
International Glaucoma Association (IGA),
Woodcote House, 15 Highpoint Business Village,
Henwood,
Ashford,
Kent, TN24 8DH.
Tel: 01233 648170
Registered Charity number 274681.
Alternatively, if you or someone you know has problems with their vision, and you require further advice or information, please phone the Royal National Institute for the Blind (RNIB) Helpline on 0303 123 9999, Monday to Friday 8.45am to 5.30pm, calls charged at local rate.
(The IGA and RNIB are independent UK charities and are not associated with Ginova Ltd or Ginova UK Ltd.)
500000/PL1a