Doxorubicin Solution For Injection 2Mg/Ml
SJPPIS' percjva|s |OiaphCS Fie | Laydown n/a
Fold 2

Peif
FoId 2
FRONT
TECHNICAL LEAFLET
Doxorubicin2 mg/ml
Solution for Injection
doxorubicin hydrochloride
abnormalities such as non-specific ST-T wave changes. Tachyarrhythmias, including premature ventricular contractions and ventricular tachycardia, bradycardia, as well as atrioventricular and bundle-branch block have also been reported. These effects do not usually predict subsequent development of delayed cardiotoxicity, and are generally not a consideration for discontinuation of doxorubicin treatment.
toxicity usually develops late in the course of therapy with doxorubicin or ination, but later events, several months to years after completion of
within 2 to 3 months after treatment termin; treatment, have also been reported. Del on (LVEF) and/or signs and
-old 5
-old 4
-old 5
-old 3
-old 5
-old 4
-old 5
2/PAR 2014-0025318 CK
08 Oct 2014
manifested by reduced left ve (CHF) such as dyspi
n and ga
IMPORTANT: Refe Presentation
Uses
nd
nd
nd
Dosage and administration
it is advisable to give the drug via a placed in the vein. This technique m
ts use within a specific treatment.
Conventional doses
When used as a single agent, the recommended starting dose of doxorubicin per cycle In adults Is 60-75 mg/m1 of body surface area. The total starting dose per cycle may be given as a single dose or divided over 3 successive days or in divided doses given on days 1 and 8.
Under conditions of normal recovery from drug-induced toxicity (particularly bone marrow depression nd stomatitis), each treatment cycle can be repeated every 3 to 4 weeks.
dependent oedema, cardiomegaly and hepatomegaly, oliguria, ascites, pleural effusion and gallop rhythm. Subacute effects such as pericarditis/myocarditis have also been reported. Life-threatening CHF is the most se form of anthracycline-induced cardiomyopathy and represents the cumulative dose-limiting toxicity of the drug. Cardiac function should be assessed before patients undergo treatment with doxorubicin and must be monitored throughout therapy to minimise the risk of incurring severe cardiac impairment. The risk may be decreased through regular monitoring of LVEF during the course of treatment with prompt discontinuation of doxorubicin at the first sign of impaired function. The appropriate quantitative method for repeated assessment of cardiac function (evaluation of LVEF) includes multi-gated radionuclide angiography (MUGA) or echocardiography (ECl A baseline cardiac evaluation with an ECG and either a MUGA scan or an ECHO is recommended, especially ii patients with risk factors for increased cardiotoxicity. Repeated MUGA or ECHO determinations of LVEF should performed, particularly with higher, cumulative anthracycline doses. The technique used for assessment shoul consistent throughout follow-up.
The probability of developing CHF, estimated around 1% to 2% at a cumulative dose of 300 mg/m1 slowly increases up to the total cumulative dose of 450-550 mg/m1. Thereafter, the risk of developing CHF increases
Risk factors for cardiac toxicity include active or dormant cardiovascular disease, prior or concomitant radioth to the mediastinal/pericardial area, previous therapy with other anthracyclines or anthracenediones and concomitant use of drugs with the ability to suppress cardiac contractility or cardiotoxic drugs (eg. trastuzum Anthracyclines including doxorubicin should not be administered in combination with other cardiotoxic agents unless the patient's cardiac function is closely monitored (see section 4.5). Patients receiving anthracyclines a stopping treatment with other cardiotoxic agents, especially those with long half-lives such as trastuzumab, n also be at an increased risk of developing cardiotoxicity. The reported half-life of trastuzumab is approximate!
28 - 38 days and may persist in the circulation for up to 27 weeks. Therefore, physicians should avoid anthracycline-based therapy for up to _ 27 weeks after stopping trastuzumab when possible. If anthracyclines a
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, please ask your doctor, pharmacist or nuise.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as youre.
If you get any side effects, tell your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
|
If it is used in combination If dosage is to be calculate |
w3i0t-h6o0thmerg/amnt2iteuvmeroyurtharegee |
|
some who believe that divi Hepatic dysfunction f hepatic function is impa |
eks greatly reduces the dis ness even though at the c |
|
Serum Bilirubin Levels |
Recommended Dose |
|
1.2 - 3.0 mg/100 ml |
50% normal dose |
|
>3.0mg/l00 ml |
25% normal dose |
y. If
Contraindications, Special warnings & precaution for use Contraindications
Doxorubicin Solution for Injection Is contra-indicated In patients with hypersensitr component of the product, other anthracyclines, or anthracenediones.
With the intravenous use (IV) of this drug, it is also contra-indicated in patients wi of cardiac impairment of the following:
• Persistent myelosuppression
• Severe myoca
• Recent myocardial infarction
• Severe arrthymias
• Previous treatment with maximum cumulative doses of doxorubicin, daunorubii Special warnings
Doxorubicin should be administered only under the supervision of physicians exp. therapy.
Patients should recover from the acute toxicities of prior cytotoxic tr thrombocytopenia, and generalized infections) before beginning tret The systemic clearance of doxorubicin is reduced in obese patients Precautions for use
During each cycle of treatment with doxorubicin Solution for Injectio
Patients should recover from the acute toxicities of prior cytotoxic tr thrombocytopenia, and generalized infections) before beginning tret The systemic clearance of doxorubicin is reduced in obese patients
Cardiac function
function must be carefully m However, cardiotoxicity with
receiving high
ur at lower cu
s and in those with whether or not card
Children and adolescents are at an increased risk for developing delayed cardiotoxicity following doxorubicin administration. Females may be at greater risk than males. Follow-up cardiac evaluations are recommended
What is in this leaflet
1. What Doxorubicin is and what it is used for
2. What you need to know before you are given Doxorubicin
3. How Doxorubicin is given to you
4. Possible side effects
5. How to store Doxorubicin
6. Contents of the pack and other information
1. What Doxorubicin is and what it is used for
• This medicine contains doxorubicin hydrochloride, which belongs to a group of medicines called cytotoxics used for chemotherapy. This medicine causes cells such as cancer cells that are actively growing, to slow or stop their growth and increases the likelihood that they die. Doxorubicin treatment helps to selectively kill the cancer tissue rather than normal, healthy tissue. It can be used in both
e are still
:h as stomatitis, neutropen
titi
nd other
Doxorubicin may produce myelosuppression. Haematologic profiles should be assessed before and during each cycle of therapy with doxorubicin, including differential white blood cell (WBC) counts. A dose-dependent, reversit
toxicity and is the most common acute dose-limiting toxicity of this drug. Leucopenia and neutropenia generaUy reach the nadir between days 10 and 14 after drug administration, the WBC/neutrophil counts return to normal values in most cases by day 21. Thrombocytopenia and anaemia may also occur. Clinical consequences of severe
adults a
• Doxorubicin is used to treat a var way in which it is used depends t
• It has been found to be particular addition, this medicine can be giv lymphomas and leukaemia.
• You must talk to a doctor if you do i
y of cancers, either alone or in combination with other drugs. The on the type of cancer that is being treated. useful in the treatment of cancers of the breast and lung. In n to treat cancers of the blood forming tissues such as malignant
tr or if you feel worse.
eath
anthracyclines. Secondary leukaemia is more common when such drugs are given in combination with DNA-damaging antineoplastic agents, when patients have been heavily pretreated with cytotoxic drugs or when
Carcinogenesis, Mutagenesis and Impairment of Fertility
Doxorubicin was genotoxic and mutagenic in vitro and in vivo tests. In women, doxorubicin may cause infertility during the time of drug administration. Doxorubicin may cause amenorrhoea Ovulation and menstruation appear to return after termination of therapy, although premature menopause can occur.
Doxorubicin is mutagenic and can induce chromosomal damage in human spermatozoa. Oligospermia or azoospermia may be permanent, however, sperm counts have been reported to return to normospermic levels in some instances. This may occur several years after the end of therapy. Men undergoing doxorubicin treatment should use effective contraceptive methods.
Liver function
The major route of elimination of doxo before and during treatment with doxo the drug with an increase in overall to 4.2). Patients with severe hepatic imp
Other
Doxorubicin may potentiate the toxicity of other anticancer therapies. Exacerbation of cyclophosphamide-indu haemorrhagic cystitis and enhanced hepatotoxicity of 6-mercaptopurine have been reported. Radiation-induce toxicities (myocardium, mucosae, skin and liver) have also been reported.
As with other cytotoxic agents, thrombophlebitis and thromboembolic phenomena including pulmonary emboli (in some cases fatal) have been coincidentally reported with the use of doxorubicin.
Tumour-Lysis Syndrome
Doxorubicin may induce hyperuricaemia as a consequence of the extensive purine catabolism that accompani drug-induced rapid lysis of neoplastic cells (tumour-lysis syndrome). Blood uric acid levels, potassium, calcium
nd creatinine should be with allopurinol to preve
2. What you need to know before you are given Doxorubicin
Do not use Doxorubicin if you have:
• If you have an allergy (hypersensitivity) to doxorubicin, other similar medicines called anthracyclines or anthracenediones or ary of the other ingredients of this medicine (listed in section 6).
• If you have low blood cell counts, as it can lower them further.
• If you have previously been treated with doxorubicin or similar chemotherapy drugs
like pharmorubicin, idarubicin, epirubicin or danuorubicin as
• If you have suffered from severe heart trouble in the past, this.
• If you have severe liver problems.
Warnings and precautions
reatment with these similar esently receiving treatment for
bicin is the hepatobilia bicin. Patients with el ity. Lower doses are r
tem. Serum total bilirubin should be ev bilirubin may experience slower clear mended in these patients (see SmPC, s orubicin (see SmPC, section 4.3).
fter
nd
Talk to your
r nur g thi
If you have or have ever had If you have had or are due to oxorubicin may also cause the
n the urin
that
sure your doc fore or during r
ur doctor will assess your nows before you start taking
d the thin layer which lines the body
lotting b
s in bl
• High levels of uric acid in the blood Refer to section 4 for further information. Other Anti-cancer Medicines
Problems are more likely to occur if you ht doses just before or at the same time as d
the anticancer drug before you begin treatment with this medicine. Your doctor will want to monitor you carefully during and after treatment (see section 3 for more information).
Other medicines and Doxorubicin
Please tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines, including medicines obtained without a prescription, particularly any of the following:
• Some medicines effect the concentration and clinical effect of doxorubicin. (e.g. verapamil, phenobarbital, phenytoin, St. John's Wort). Please tell your doctor or pharmacist if you are taking any of these medicines.
• Cyclosporine: which can make the effects of doxorubicin stronger and may result in prolonged decrease in bone marrow and blood cells (coma and seizures have also been described with concomitant administration of cyclosporine and doxorubicin).
• Calcium Channel Blockers: medicines for your heart.
• Sorafenib: used to treat inoperable liver cancer and advanced kidney cancer.
• Paclitaxel: which can make the effects of doxorubicin stronger Pregnancy
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before being given this medicine because it may cause birth defects.
If you are sexually active, you are advised to use effective birth control to prevent pregnancy during treatment, whether you are male or female.
Breast-feeding
You should stop breast-feeding before starting treatment with this medicine as some of the drug may get into your breast milk and possibly harm your child.
Driving and using machines
There are no special precautions and you can drive and operate machinery as long as you feel fully recovered following your hospital treatment.
Doxorubicin contains sodium
This medicinal product contains less than 1mmol sodium (23mg) per dose, i.e. essentially sodium free.
3. How Doxorablcln is given to you
If you are prescribed doxorubicin it will only be given to you by doctors or nurses experienced in giving chemotherapy.
This medicine will be given to you by a doctor or nurse through a drip (infusion) into a vein. Your doctor will decide what dose to give and the number of days treatment you will receive depending on your condition.
The dose is decided by taking into account the condition you have, your height and weight. From your height and weight the doctor will work out your body surface area; and it is this that your dose is calculated from.
While one course of treatment may sometimes be enough, more often your doctor will advise further courses in either one, three or four weeks time. It may take several courses before your illness is under control and you feel better.
Regular checks by your doctor during your treatment with Doxorubicin solution
During treatment your doctor will be making regular checks of your:
• Blood: To check for low blood cell counts that may need treatment.
• Heart Function: Heart damage can occur when high doses of doxorubicin are given. This may not be detected for several weeks; so regular tests may be required during this period.
• Liver: Using blood tests, your doctor will check that this medicine is not affecting the way it functions in a harmful way.
• Blood uric acid levels: Doxorubicin may increase uric acid levels in the blood which might cause gout. Another medicine may be given if your uric acid levels are too high.
If you receive high doses of Doxorubicin
High doses can worsen side effects like sores in the mouth or may decrease the number of white blood cells (which fight infection) and platelets (these help the blood to clot) in the blood. Should this happen, you may need antibiotics or blood transfusions. Mouth ulcers can be treated to make them less uncomfortable as they heal.
4. Possible side effects
Like all medicines, this medicine can have side effects although not everybody gets them.
Please contact your doctor or nurse immediately if you notice any of the following side effects:
• Feeling dizzy, feverish, short of breath with a tight chest or throat or have an itchy rash. This type of allergic reaction can be very serious.
• Anaemia (a low red blood cell count) that can leave you feeling tired and lethargic.
• White blood cell counts (which fight infection) can also drop, increasing the chance of infections and a raised temperature (fever).
• Platelets (these are cells that help the blood to clot) can be affected which could make you bruise or bleed more easily. It is important to seek medical advice if this happens. Your doctor should test your blood cell count during treatment.
• Doxorubicin may also cause decreased activity in your bone marrow.
u will
er medicines especially at high time to recover from the effects of
Continued overleaf...
DRUK DX PAM644
PFIZER (PERTH) PTY LIMITED, AUSTRALIA
PAR-2014-0025318 DRAFT# 1 08 Oct 2014
BACK
Other side effects that may occur are as follows:
Very common: may affect more than 1 in 10 people
• Infection
• Lack of appetite.
• Inflammation in the mouth, diarrhoea, feeling sick (nausea) being sick (vomiting).
• Reddening, swelling, numbness, pain and tingling in the palms and feet may also occur whilst being treated with doxorubicin.
• Hair loss is common and may be quite severe. Beard growth may stop in men. Hair normally re-grows when your treatment course ends.
• Fever, feeling weak, chills.
• Abnormal ECG (this is an electrical trace of your heart) results.
• Raised levels of liver erzymes (as detected by a blood test) can determine if the medicine is having an abnormal effect on your liver.
• Weight increased in patients with early breast cancer.
Common: may affect up to 1 in 10 people
• Blood poisoning.
• You may notice your heart beating abnormally quickly, with an increase in pulse rate. In some cases, you may notice heart problems several months or years after medication has been completed.
• Conjunctivitis (usually causing red watery eyes), excess tear production.
• Heart failure which can be associated with the symptoms of shortness of breath and swelling of the ankles.
• Increased heart rate, inflammation of the throat and gullet, stomach pain, skin rashes, redness, hives, nails and skin may appear darker than usual.
• Redness and swelling may develop at site of injection.
Uncommon: may affect up to 1 in 100 people
• Embolism (a blockage in the bloodstream).
Not known: (frequency cannot be estimated from the available data)
• Dehydration, increased uric acid in your urine, inflamed cornea, watery eyes, general discomfort.
• Increased heart rate, chest pain which could indicate heart problems, shock (low blood pressure and circulation), internal bleeding.
• Inflammation of veins, blockage of a blood vessel by a clot (thromboembolism), hot flushes.
• Irritation or bleeding in the intestines, inflammation of the lining of the stomach, heartburn, soreness or ulcers in the mouth, which may not appear until 3-10 days after treatment, discoloration inside the mouth.
• Increased sensitivity of the skin to the sunlight
• Inflammation reaction, which could occur soon after treatment or years later, itchy skin and other skin disorders.
• Reddening of your urine, (which is normal and related to the colour of the medicine). You should inform your doctor if it does not stop in a few days or you think there is blood in your urine. Let your doctor know if you get these symptoms.
• In women, doxorubicin may cause infertility during the time the drug is taken. Women may also find that their periods stop, but their periods should return to normal after medication is stopped. In some cases early menopause can occur.
In men, doxorubicin may cause an absence or decrease in sperm count, but this may return to normal after medication is stopped. Both men and women taking doxorubicin should use effective contraceptive methods.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects via the Yellow Card Scheme website: www.mhra.gov.uk/vellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Doxorubicin
• The unopened vials should be stored in the original container in a fridge until ready for use.
• Keep out of the sight and reach of children.
• This medicine should not be used after the expiry date printed on the box and on the vial after EXP. The expiry date refers to the last day of that month. The pharmacist will check this when your medicine is prepared for you. If the solution is cloudy after preparation, the pharmacist will dispose of it safely.
6. Contents of the pack and other information
What Doxorubicin contains
The active substance is doxorubicin hydrochloride. The other ingredients are sodium chloride, water for injections and hydrochloric acid.
What Doxorubicin looks like and contents of the pack
Doxorubicin solution for injection is a red liquid in single plastic vials containing 2mg/ml of the active ingredient, doxorubicin hydrochloride.
Marketing Authorisation Holder
Pfizer Limited Ramsgate Road Sandwich Kent
CT13 9NJ United Kingdom
Manufacturer:
Pfizer Service Company
BVBA
Zaventem
Belgium
Company Contact Address:
For further information on your medicine please contact Medical Information at Pfizer Limited, Walton Oaks, Tadwoith, Surney, KT20 7NS, UK.
Tel :01304 616161
This leaflet was last revised 09/2014
Ref: DO 14_0
ZZZ000ZZ

Reporting ol suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows report any suspected adverse reactions via the Yellowed . .
ry. It is d. This pro
ed to have ha
d as it will result in hydrolysis of the
but it is not recommended that it be infusion bag or at the Y-site of an IV
Vaccinations
Administration ot live or live-attenuated vaccines In patients Immunocompromised by chemotherapeutic agents, Including doxorubicin, may result in serious or fatal infections. Vaccination with a live vaccine should be avoided in patients receiving doxorubicin. Killed or inactivated vaccines may be administered; however, the response to such vaccines may be diminished.
Interactions
Doxorubicin is a major substrate of cytochrome P450 CYP3A4 and CYP2D6, and P-glycoprotein (P-gp). Clinically significant interactions have been reported with inhibitors of CYP3A4, CYP2D6, and/or P-gp (eg. verapamil), resulting in increased concentration and clinical effect of doxorubicin. Inducers of CYP3A4 (e g. phenobarbital, phenytoin, St. John's Wort) and P-gp inducers may decrease the concentration of doxorubicin.
The addition of cyclosporine to doxorubicin may result in increases in area under the concentration-time curve (AUC) for both doxorubicin and doxorubicinol, possibly due to a decrease in clearance of the parent drug and a decrease in metabolism of doxorubicinol. Literature reports suggest that adding cyclosporine to doxorubicin results in more profound and prolonged haematologic toxicity than that observed with doxorubicin alone. Coma and seizures have also been described with concomitant administration of cyclosporine and doxorubicin.
High dose cyclosporine increases the serum levels and myelotoxicity of doxorubicin.
Doxorubicin is mainly used in combination with other cytotoxic drugs. Additive toxicity may occur especially with regard to bone marrow/haematologic and gastrointestinal effects (see section 4.4 Special Warnings and Precautions for Use). The use of doxorubicin in combination chemotherapy with other potentially cardiotoxic drugs, as well as the concomitant use of other cardioactive compounds (eg. calcium channel blockers), require monitoring of cardiac function throughout treatment. Changes in hepatic function induced by concomitant therapies may affect doxorubicin metabolism, pharmacokinetics, therapeutic efficacy and/or toxicity Paclitaxel can cause increased plasma-concentrations of doxorubicin and/or its metabolites when given prior to doxorubicin. Certain data indicate that a smaller increase is observed when doxorubicin is administered prior to paclitaxel.
In a clinical study, an increase in doxorubicin AUC of 21% was observed when given with sorafenib 400 mg daily The clinical significance of this finding is unknown.
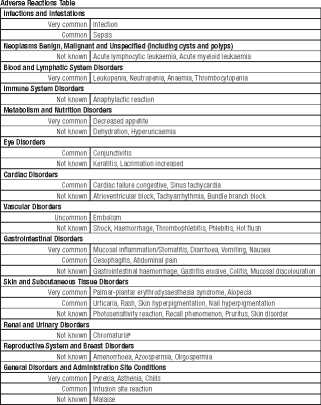
Adverse Reactions
Apart from the precaution described above, the following adverse reactions have been described:
Adverse reactions reported in association with doxorubicin therapy are listed below by MedDRA System Organ Class and by frequency. Frequencies are defined as: Very common £10%), Common (>1%, <10%), Uncommon £0.1%, <1%), Rare (>0.01%, <0.1%), Very rare (<0.01%), and Not known (cannot be estimated from available
any suspected adverse reactions via the Yellow Card Scheme at www.ml Fertility, pregnancy and lactation
Doxorubicin should not be used during pregnancy unless clearly neces pharmacological effects on pregnancy and/or the foetus/newborn ch administered to patients who are pregnant or to mothers who are br
Overdose
Very high single doses of doxorubicin may be expected to cause acute myocardial degeneration within 24 ho and severe myelosuppression within 10-15 days. Treatment should aim to support the patient during this per and should utilise such measures as blood transfusion and reverse barrier nursing.
Delayed cardiac failure has been seen with the anthracyclines up to 6 months after the overdose, if signs of cardiac failure arise, they should be treated along conventional lines. 2 1
2/PAR 2014-0025318 CK
08 Oct 2014
years.
Storage
The vials should be stored at between 2° - 8° C (in the refrigerator.)
Package Quantities
10 mg, 20 mg, 50 mg and 200 mg vials.Not all pack sizes may be marketed.
| POM |
PL 00057/0970
This leaflet was prepared in 09/2014 Ref: DO 14_0
Further information is available to the medical and allied professions on request from:
Medical Information at Pfizer Limited, Walton Oaks, Tadworth, Surrey, KT20 7NS, UK.
Tel: 01 304 616161
ZZZ000ZZ
The following protective recommendations are given due to the toxic nature of this substance: Personnel should be trained in good technique for handling.
Pregnant staff should be excluded from working with this drug.
Personnel handling doxorubicin Solution should wear protective clothing: goggles, gowns, and dis gloves and masks.
All items used for administration or cleaning, including gloves, should be placed in high-risk, was bags for high temperature incineration.
Spillage or leakage should be treated with dilute sodium hypochlorite (1% available chlorine) solu preferably by soaking, and then water.
All cleaning materials should be disposed of as indicated previously.
Accidental contact with the skin or eyes should be treated immediately by copious lavage with w and water, or sodium bicarbonate solution; medical attention should be sought.
Discard any unused solution.
Incompatibilities
Prolonged contact with any solution of an alkaline pH shoi Doxorubicin should not be mixed with heparin due to chei when the drugs are in certain proportions.
Doxorubicin can be used in combination with other antitu mixed with other drugs.
Doxorubicin should not be mixed with fluorouracil (eg., in the same IV infusion bag or infusion line) since it has been reported that these drugs are incompatible to the exter form. If concomitant therapy with doxorubicin and fluorouracil is required, it is recomn flushed between the administration of these drugs.
Shelf life