Duosol Without Potassium Solution For Haemofiltration
SUMMARY OF PRODUCT CHARACTERISTICS
1. NAME OF THE MEDICINAL PRODUCT
Duosol without Potassium solution for haemofiltration
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
|
Small Chamber Electrolyte Solution |
Large Chamber Bicarbonate Solution | |||
|
Active substances: |
555 ml contain |
per 1000 ml |
4445 ml contain |
per 1000 ml |
|
Sodium chloride |
2.34 g |
4.21 g |
27.47 g |
6.18 g |
|
Calcium chloride dihydrate |
1.10 g |
1.98 g |
— |
— |
|
Magnesium chloride hexahydrate |
0.51 g |
0.91 g |
— |
— |
|
Glucose monohydrate equivalent to glucose anhydrous |
5.49 g 5.0 g |
9.90 g 9.0 g |
— |
— |
|
Sodium hydrogen carbonate |
— |
— |
15.96 g |
3.59 g |
|
Electrolytes: |
[mmol/ chamber] |
[mmol/l] |
[mmol/ chamber] |
[mmol/l] |
|
Na+ |
40.0 |
72 |
660 |
149 |
|
Ca2+ |
7.5 |
13.5 |
— |
— |
|
Mg2+ |
2.5 |
4.5 |
— |
— |
|
Cl- |
75.0 |
135 |
470 |
106 |
|
HCO3" |
— |
— |
190 |
42.8 |
|
Theoretical osmolarity [mOsm/l] |
275 |
297 | ||
Composition of the ready-to-use solution for haemofiltration after mixing:
1000 ml ready-to-use solution for haemofiltration contain [mmol/l]:
Na+
Ca2+
Mg2+
Cl-
HCO3-
Glucose anhydrous
140
1.5 0.5 109 35.0
5.6 (equiv. to 1.0 g)
For the full list of excipients, see section 6.1.
3. PHARMACEUTICAL FORM
Solution for haemofiltration
Clear and colourless solution, free from visible particles
Theoretical osmolarity: 292 mOsm/l pH: 7.0-8.0
4. CLINICAL PARTICULARS
4.1 Therapeutic indications
The ready-to-use solution is indicated for use in patients with acute renal failure of any cause requiring continuous haemofiltration.
The use of solutions for haemofiltration in patients with acute renal failure should be under the direction of a physician with experience in using such treatment.
Posology
The filtration rate prescribed is dependent on the clinical status and the body weight of the patient. Unless otherwise prescribed, a filtration rate of 20-25 ml/kg body weight per hour is recommended for the removal of metabolic waste products normally excreted in the urine, depending on the metabolic condition of the patient.
The dose-volume is at the discretion of the physician because the volume of substitution solution depends on the intensity of treatment performed and on the amount of fluid to be replaced in order to achieve fluid balance.
Paediatric population
The dosage recommendations mentioned above are also applicable for the paediatric population.
Method of administration
Intravenous use.
The ready-to-use solution for haemofiltration has to be prepared by opening the peel seam. The mixing is performed by twisting the bag five times. For further instructions, see section 6.6.
The ready-to-use solution for haemofiltration is infused into the extracorporeal circulation by means of an infusion pump.
During haemofiltration the solution for haemofiltration replaces the ultrafiltrate removed from the blood taking into account overall fluid balance.
In acute renal failure, treatment is carried out for a limited time period and is discontinued when renal function is fully restored.
4.3 Contraindications
Specific to the ready-to-use solution for haemofiltration:
• Hypokalaemia
• Metabolic alkalosis
For haemofiltration in general:
• Acute renal failure with a marked hypercatabolic state when uraemic symptoms can no longer be corrected by haemofiltration
• Inadequate blood flow from the vascular access
• All states of increased risk of haemorrhage due to systemic anticoagulation
4.4 Special warnings and precautions for use
The haemodynamic status, fluid balance, electrolyte and acid-base balance, blood glucose, and levels of urea and plasma creatinine should be closely monitored before and during haemofiltration.
The serum potassium concentration must be regularly monitored before and during haemofiltration. If the serum potassium falls and hypokalaemia develops, supplementation of potassium and/or changing to a substitution solution with higher potassium concentration may become necessary. In cases of increased serum potassium, hyperkalaemia, an increase in the filtration rate may be indicated together with the usual measures of intensive care medicine.
The inorganic phosphate concentration should be measured regularly during haemofiltration. Inorganic phosphate must be substituted in cases of hypophosphataemia.
Plastic containers are occasionally damaged during transport from the manufacturer to the hospital/dialysis unit or within the hospital/dialysis unit. This can lead to contamination with microbial or fungal growth in the solution for haemofiltration. Therefore, careful visual inspection of the container and the solution for haemofiltration is necessary before attaching the container and before administration of the solution. Particular attention should be paid to the slightest damage to the closure, to the preparation seal, to the peel seam and to the corners of the container as sources of possible contamination.
The solution for haemofiltration must only be used if the container (outer wrap and two-chamber bag), peel seam and connectors are undamaged and intact and if the solution is clear and colourless and free from visible particles. The solution must only be used after opening the peel seam and mixing of the two solutions. For further instructions, see section 6.6.
If in doubt the decision concerning the use of the solution should be made by the physician in charge of the treatment.
The solution for haemofiltration should be warmed to approximately body temperature by an integrated or external heater. The solution must not be infused under any circumstances if below room temperature.
During application of this medicinal product, white calcium carbonate precipitation has been observed in the tubing lines in rare cases, particularly close to the pump unit and the heating unit. Therefore, the solution in the tubing lines should be closely visually inspected every 30 min during haemofiltration in order to ensure that the solution in the tubing system is clear and free from precipitate. Precipitations may occur also with substantial delay after start of treatment. If precipitate is observed, the solution and the tubing lines must be replaced immediately and the patient carefully monitored.
4.5 Interaction with other medicinal products and other forms of interaction
The blood concentration of filterable medicinal products, e.g. medicinal products with low protein binding capacity, may be reduced during treatment and corresponding corrective therapy should be instituted if necessary.
Interactions with other medicinal products can be avoided by correct dosing of the solution for haemofiltration and strict monitoring of clinical chemistry parameters and vital signs.
However, the following interactions are conceivable:
• Electrolyte substitutions, parenteral nutrition and other infusions usually given in intensive care medicine interact with the serum composition and the fluid status of the patient. This must be considered when prescribing haemofiltration treatment.
• Toxic effects of digitalis may be masked by hyperkalaemia, hypermagnesaemia and hypocalcaemia. The correction of these electrolytes by haemofiltration may precipitate signs and symptoms of digitalis toxicity, e.g. cardiac arrhythmia. If potassium levels are low or calcium levels high, digitalis toxicity may occur at sub-optimal doses of digitalis therapy.
• Vitamin D and medicinal products containing calcium, e.g. calcium carbonate as phosphate binder, can increase the risk of hypercalcaemia.
• Additional sodium bicarbonate substitution can increase the risk of metabolic alkalosis.
4.6 Fertility, pregnancy and lactation
Pregnancy
There are no data from the use of Duosol in pregnant women or from animal studies. However, because all the ingredients of the solution for haemofiltration are physiological substances that serve to replace essential plasma components removed by haemofiltration, no risks for the unborn child are to be expected. The use of Duosol may be considered during pregnancy, if necessary.
Lactation
Because all the ingredients of the solution for haemofiltration are physiological substances that serve to replace essential plasma components removed by haemofiltration, no risks for the child are to be expected. The use of Duosol may be considered during lactation, if necessary.
Fertility
Because all the ingredients of the solution for haemofiltration are physiological substances that serve to replace essential plasma components removed by haemofiltration, no effects on fertility are to be expected.
4.7 Effects on ability to drive and use machines
Not relevant.
4.8 Undesirable effects
There have been no reports of adverse reactions that might possibly be associated with the bicarbonate-buffered solution for haemofiltration. However, the following adverse reactions could result from the treatment or the solution used. The frequency of these adverse reactions is not known (cannot be estimated from the available data):
Metabolism and nutrition disorders
Hyper- or dehydration, electrolyte disturbances, hypophosphataemia, hyperglycaemia, metabolic alkalosis
Vascular disorders Hypertension, hypotension
Gastrointestinal disorders Nausea, vomiting
Musculoskeletal and connective tissue disorders Muscle cramps
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at www.mhra.gov .uk/yellowcard.
4.9 Overdose
Following the use of recommended doses no reports of emergency situations have arisen; moreover, the administration of the solution can be discontinued at any time. If fluid balance is not accurately calculated and monitored, hyperhydration or dehydration may occur, manifest through changes in blood pressure, central venous pressure, heart rate and pulmonary arterial pressure.
In cases of hyperhydration, ultrafiltration should be increased and the rate and volume of solution for haemofiltration infused should be reduced.
In cases of severe dehydration, ultrafiltration should be decreased or discontinued and the volume of solution for haemofiltration infused should be increased as appropriate.
Bicarbonate overdose can occur if an inappropriately large volume of solution for haemofiltration is administered and this might lead to metabolic alkalosis, decrease of ionised calcium or tetany.
Overtreatment can cause congestive cardiac failure and/or pulmonary congestion and may result in disturbances in electrolyte concentrations and acid-base balance.
5. PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Hemofiltrates, ATC code: B05ZB Basic principles of haemofiltration
Water and dissolved substances, such as uraemic toxins, electrolytes and bicarbonate, are removed from the blood by ultrafiltration during the process of continuous haemofiltration. The ultrafiltrate is replaced by a solution for haemofiltration with balanced concentrations of electrolytes and buffer.
The ready-to-use solution consisting of a bicarbonate solution and an electrolyte solution is a mixed bicarbonate-buffered solution for haemofiltration for treatment of acute renal failure by means of continuous haemofiltration.
The electrolytes Na+, K+, Mg2+, Ca2+, Cl" and bicarbonate are essential for maintaining and correcting fluid and electrolyte homeostasis (blood volume, osmotic equilibrium, acid-base balance).
The efficacy of comparable intravenously administered solutions for maintenance of the acid-base equilibrium during haemofiltration has been demonstrated unequivocally in trials and many years of clinical application. It has been confirmed that they are safe and well tolerated. The pharmacology of intravenously administered electrolytes and bicarbonate is adequately understood.
5.2 Pharmacokinetic properties
The ready-to-use solution for haemofiltration is for intravenous administration. The distribution of electrolytes and bicarbonate depends on the requirement, metabolic conditions and residual renal function. With the exception of glucose, the ingredients of the solution for haemofiltration are not subject to metabolism. The excretion of water and electrolytes depends on the cellular requirement, metabolic state, residual renal function and fluid losses e.g. via the gut, lungs and skin.
5.3 Preclinical safety data
Toxicological studies have not been carried out since all the ingredients of the solution for haemofiltration are physiological substances that serve to replace essential plasma components removed by haemofiltration.
6. PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Electrolyte solution (small chamber)
Hydrochloric acid 25% (for pH adjustment)
Water for injections
Bicarbonate solution (large chamber)
Carbon dioxide (for pH adjustment)
Water for injections
6.2 Incompatibilities
In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products. If an addition of medicinal product to the solution for haemofiltration is required, this should be carried out only after full evaluation of its compatibility with the solution for haemofiltration and only after the two solutions in the two-chamber bag have been thoroughly mixed.
6.3 Shelf life
Shelf life in the undamaged container 2 years
Shelf life after preparation of the ready-to-use solution
The mixed product should be used immediately. The mixed product is physically and chemically stable for 24 hours at 25 °C.
6.4 Special precautions for storage
Do not store above 30 °C.
Do not refrigerate or freeze.
6.5 Nature and contents of container
Polypropylene-based (PP) two-chamber bag in a PP-based outer wrap, containing 4445 ml bicarbonate solution and 555 ml electrolyte solution separated by a peel seam, with two PP-based tubes sealed by polycarbonate-based Luer-Lock connectors on the large chamber. The tube on the small chamber is used in production only and not intended for use.
2 bags of 5000 ml (two-chamber bags, 4445 ml and 555 ml) per carton
6.6 Special precautions for disposal and other handling
Instructions for preparation of the ready-to-use solution for haemofiltration
The container and the solution must be visually inspected prior to use. The solution for haemofiltration must only be used if the container (outer wrap and two-chamber bag), peel seam and connectors are undamaged and intact and if the solution is clear and colourless and free from visible particles.
Remove the outer wrap only immediately before use.
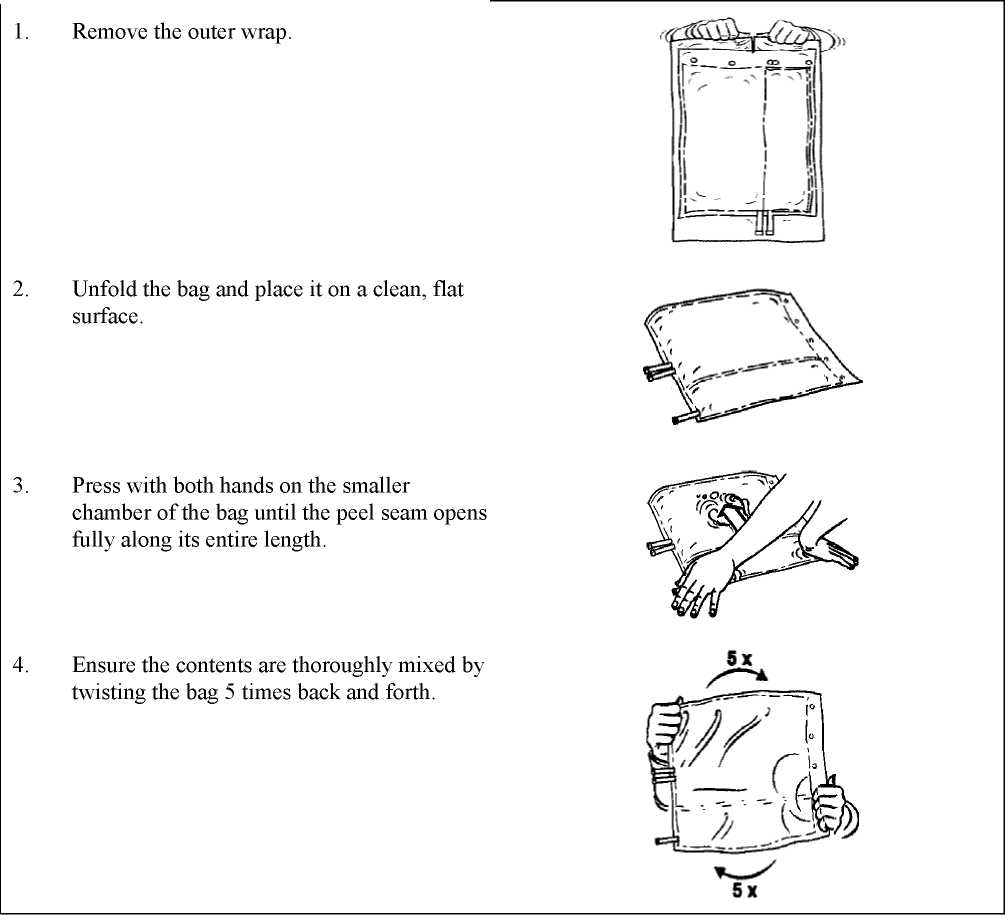
For single use only. Any unused portion of solution and any damaged containers must be discarded.
7. MARKETING AUTHORISATION HOLDER
B. Braun Avitum AG Schwarzenberger Weg 73-79 34212 Melsungen Germany
8. MARKETING AUTHORISATION NUMBER(S)
PL 22162/0001
9. DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 01/08/2006 Date of latest renewal: 19/09/2009
10 DATE OF REVISION OF THE TEXT
02/10/2015
7