Dysport 500 Units Powder And Solvent For Solution For Injection
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Dysport.
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Clostridium botulinum type A toxin-haemagglutinin complex 500 units*
* One unit (U) is defined as the median lethal intraperitoneal dose in mice.
For a full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Injection.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Dysport is indicated for symptomatic treatment of focal spasticity of:
- Upper limbs in adults
- Dynamic equinus foot deformity in ambulant paediatric cerebral palsy patients, two years of age or older.
Dysport is indicated in adults for symptomatic treatment of:
- Spasmodic torticollis
- Blepharospasm
- Hemifacial spasm.
4.2 Posology and method of administration
The units of Dysport are specific to the preparation and are not interchangeable with other preparations of botulinum toxin.
Training: Dysport should only be administered by appropriately trained physicians. Ipsen can facilitate training in administration of Dysport injections
For instructions on reconstitution of the powder for solution for injection, handling and disposal of vials please refer to section 6.6.
Focal spasticity affecting the upper limbs
Posology
Dosing in initial and sequential treatment sessions should be tailored to the individual based on the size, number and location of muscles involved, severity of spasticity, the presence of local muscle weakness, the patient's response to previous treatment, and/or adverse event history with Dysport. In clinical trials, doses of 500 Units and 1000 Units were divided among selected muscles at a given treatment session as shown below.
No more than 1 mL should generally be administered at any single injection site.
|
Muscles Injected |
Recommended Dose DYSPORT (U) |
|
Flexor carpi radialis (FCR) |
100-200 U |
|
Flexor carpi ulnaris (FCU) |
100-200 U |
|
Flexor digitorum profundus (FDP) |
100-200 U |
|
Flexor digitorum superficialis (FDS) |
100-200 U |
|
Flexor Pollicis Longus |
100-200 U |
|
Adductor Pollicis |
25-50 U |
|
Brachialis |
200-400 U |
|
Brachioradialis |
100-200 U |
|
Biceps Brachii (BB) |
200-400 U |
|
Pronator Teres |
100-200 U |
|
Triceps Brachii (long head) |
150-300 U |
|
Pectoralis Major |
150-300 U |
|
Subscapularis |
150-300 U |
|
Latissimus Dorsi |
150-300 U |
Although actual location of the injection sites can be determined by palpation the use of injection guiding technique, e.g. electromyography, electrical stimulation or ultrasound is recommended to target the injection sites.
Clinical improvement may be expected one week after injection and may last up to 20 weeks. Injections may be repeated every 12-16 weeks or as required to maintain response, but not more frequently than every 12 weeks. The degree and pattern of muscle spasticity at the time of re-injection may necessitate alterations in the dose of Dysport and muscles to be injected.
Children: The safety and effectiveness of Dysport in the treatment of upper limb spasticity in children have not been demonstrated.
Elderly patients (> 65 years): Clinical experience has not identified differences in response between the elderly and younger adult patients. In general, elderly patients should be observed to evaluate their tolerability of Dysport, due to the greater frequency of concomitant disease and other drug therapy.
Method of administration
When treating focal spasticity affecting the upper limbs in adults, Dysport is reconstituted with sodium chloride injection B.P. (0.9% w/v) to yield a solution containing either 100 units per mL, 200 units per mL or 500 units per mL of Dysport (see section 6.6).
Dysport is administered by intramuscular injection into the muscles as described above.
Dynamic equinus foot deformity due to focal spasticity:
Posology
The initial recommended dose is 20 units/kg body weight given as a divided dose between both calf muscles. If only one calf is affected, a dose of 10 units/kg body weight should be used.
Consideration should be given to lowering this starting dose if there is evidence to suggest that this dose may result in excessive weakness of the target muscles, such as for patients whose target muscles are small or patients who require concomitant injections to other muscle groups.
Following evaluation of response to the starting dose subsequent treatment may be titrated within the range 10 units/kg and 30 units/kg divided between both legs. The maximum dose administered must not exceed 30 units/kg or 1000 units, whichever is the lower. Administration should primarily be targeted to the gastrocnemius, although injections of the soleus and injection of the tibialis posterior should also be considered.
The use of electromyography (EMG) is not routine clinical practice but may assist in identifying the most active muscles.
Clinical improvement may be expected within two weeks after injection. Injections may be repeated approximately every 16 weeks or as required to maintain response, but not more frequently than every 12 weeks.
Method of administration
When treating paediatric cerebral palsy spasticity, Dysport is reconstituted with sodium chloride injection B.P. (0.9% w/v) to yield a solution containing 500 units per mL of Dysport (see section 6.6).
Dysport is administered by intramuscular injection into the calf muscles as described above.
Spasmodic torticollis:
Posology
The doses recommended for torticollis are applicable to adults of all ages, provided the adults are of normal weight with no evidence of reduced neck muscle mass. A lower dose may be appropriate if the patient is markedly underweight or in the elderly, where reduced muscle mass may exist.
The initial recommended dose for the treatment of spasmodic torticollis is 500 units per patient given as a divided dose and administered into the two or three most active neck muscles.
• For rotational torticollis distribute the 500 units by administering 350 units into the splenius capitis muscle, ipsilateral to the direction of the chin/head rotation and 150 units into the sternomastoid muscle, contralateral to the rotation.
• For laterocollis, distribute the 500 units by administering 350 units into the ipsilateral splenius capitis muscle and 150 units into the ipsilateral sternomastoid muscle. In cases associated with shoulder elevation the ipsilateral trapezoid or levator scapulae muscles may also require treatment, according to visible hypertrophy of the muscle or electromyographic (EMG) findings.
Where injections of three muscles are required, distribute the 500 units as follows, 300 units splenius capitis, 100 units sternomastoid and 100 units to the third muscle.
• For retrocollis distribute the 500 units by administering 250 units into each of the splenius capitis muscles. Bilateral splenii injections may increase the risk of neck muscle weakness.
• All other forms of torticollis are highly dependent on specialist knowledge and EMG to identify and treat the most active muscles. EMG should be used diagnostically for all complex forms of torticollis, for reassessment after unsuccessful injections in non complex cases, and for guiding injections into deep muscles or in overweight patients with poorly palpable neck muscles.
On subsequent administration, the doses may be adjusted according to the clinical response and side effects observed. Doses within the range of 250-1000 units are recommended, although the higher doses may be accompanied by an increase in side effects, particularly dysphagia. The maximum dose administered must not exceed 1000 units.
The relief of symptoms of torticollis may be expected within a week after the injection.
Injections may be repeated approximately every 16 weeks or as required to maintain a response, but not more frequently than every 12 weeks.
Children: The safety and effectiveness of Dysport in the treatment of spasmodic torticollis in children have not been demonstrated.
Method of administration
When treating spasmodic torticollis Dysport is reconstituted with sodium chloride injection B.P. (0.9% w/v) to yield a solution containing 500 units per mL of Dysport (see section 6.6).
Dysport is administered by intramuscular injection as described above.
Blepharospasm and hemifacial spasm:
Posology
In a dose ranging clinical trial on the use of Dysport for the treatment of benign essential blepharospasm, a dose of 40 units per eye was significantly effective. Doses of 80 units and 120 units per eye resulted in a longer duration of effect. However, the incidence of local adverse events, specifically ptosis, was dose related. In the treatment of blepharospasm and hemifacial spasm, the maximum dose used must not exceed a total dose of 120 units per eye.
An injection of 10 units (0.05 ml) medially and 10 units (0.05 ml) laterally should be made into the junction between the preseptal and orbital parts of both the upper (3 and 4) and lower orbicularis oculi muscles (5 and 6) of each eye. In order to reduce the risk of ptosis, injections near the levator palpebrae superioris should be avoided.
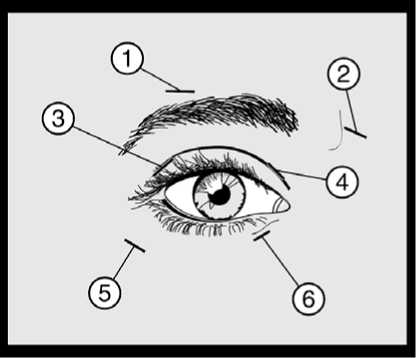
For injections into the upper lid the needle should be directed away from its centre to avoid the levator muscle. A diagram to aid placement of these injections is provided above. The relief of symptoms may be expected to begin within two to four days with maximal effect within two weeks.
Injections should be repeated approximately every twelve weeks or as required to prevent recurrence of symptoms but not more frequently than every twelve weeks.
On such subsequent administrations, if the response from the initial treatment is considered insufficient, the dose per eye may need to be increased to 60 units: 10 units (0.05mL) medially and 20 units (0.1mL) laterally, 80 units: 20 units (0.1mL) medially and 20 units (0.1mL) laterally or up to 120 units: 20 units (0.1mL) medially and 40 units (0.2mL) laterally above and below each eye in the manner previously described. Additional sites in the frontalis muscle above the brow (1 and 2) may also be injected if spasms here interfere with vision.
In cases of unilateral blepharospasm the injections should be confined to the affected eye. Patients with hemifacial spasm should be treated as for unilateral blepharospasm. The doses recommended are applicable to adults of all ages including the elderly.
Children: The safety and effectiveness of Dysport in the treatment of blepharospasm and hemifacial spasm in children have not been demonstrated.
Method of administration
When treating blepharospasm and hemifacial spasm, Dysport is reconstituted with sodium chloride injection B.P. (0.9% w/v) to yield a solution containing 200 units per mL of Dysport (see section 6.6).
Dysport is administered by subcutaneous injection medially and laterally into the junction between the preseptal and orbital parts of both the upper and lower orbicularis oculi muscles of the eyes as described above.
4.3 Contraindications
Dysport is contraindicated in individuals with known hypersensitivity to any components of Dysport.
4.4 Special warnings and precautions for use
Side effects related to spread of toxin distant from the site of administration have been reported (see section 4.8) which, in some cases, was associated with dysphagia, pneumonia and/or significant debility resulting, very rarely, in death. Patients treated with therapeutic doses may present with excessive muscle weakness. The risk of occurrence of such undesirable effects may be reduced by using the lowest effective possible dose and by not exceeding the maximum recommended dose.
Dysport should only be used with caution and under close medical supervision in patients with subclinical or clinical evidence of marked defective neuromuscular transmission (e.g. myasthenia gravis). Such patients may have an increased sensitivity to agents such as Dysport, which may result in excessive muscle weakness with therapeutic doses. Patients with underlying neurological disorders are at increased risk of this side effect.
Very rare cases of death, occasionally in the context of dysphagia, pneumopathy (including but not limited to dyspnoea, respiratory failure, respiratory arrest) and/or in patients with significant asthenia have been reported following treatment with botulinum toxin A or B. Patients with disorders resulting in defective neuromuscular transmission, difficulty in swallowing or breathing are more at risk of experiencing these effects. In these patients, treatment must be administered under the control of a specialist and only if the benefit of treatment outweighs the risk.
Dysport should be administered with caution to patients with pre-existing swallowing or breathing problems as these can worsen following the distribution of the effect of toxin into the relevant muscles. Aspiration has occurred in rare cases and is a risk when treating patients who have a chronic respiratory disorder.
The recommended posology and frequency of administration for Dysport must not be exceeded (see section 4.2).
Patients and their care-givers must be warned of the necessity to seek immediate medical treatment in case of swallowing, speech or respiratory problems.
For the treatment of spasticity associated with cerebral palsy in children, Dysport should only be used in children of 2 years of age or over.
Dysport should not be used to treat spasticity in patients who have developed a fixed contracture.
As with any intramuscular injection, Dysport should only be used where strictly necessary in patients with prolonged bleeding times, infection or inflammation at the proposed site(s) of injection.
Dysport should only be used to treat a single patient, during a single session. Specific precautions must be taken during the preparation and administration of the product (see section 4.2) and for the inactivation and disposal of any unused reconstituted solution (see section 6.6).
Antibody formation to botulinum toxin has been noted rarely in patients receiving Dysport. Clinically, neutralising antibodies might be suspected by a substantial deterioration in response to therapy and/or the need for consistent use of increased doses.
Careful consideration should be given before the injection of patients who have experienced a previous allergic reaction to a product containing botulinum toxin type A. The risk of a further allergic reaction must be considered in relation to the benefit of treatment.
4.5. Interaction with other medicinal products and other forms of interaction
The effects of botulinum toxin may be potentiated by drugs interfering either directly or indirectly with neuromuscular function (e.g. aminoglycosides, curare-like nondepolarising blockers) and such drugs should be used with caution in patients treated with botulinum toxin.
4.6 Pregnancy and lactation
Pregnancy:
There are limited data from the use of Clostridium botulinum type A toxin-haemagglutinin complex in pregnant women. Studies in animals have shown reproductive toxicity at high doses causing maternal toxicity (see section 5.3).
Dysport should be used during pregnancy only if the benefit justifies any potential risk to the fxtus. Caution should be exercised when prescribing to pregnant women.
Lactation:
It is not known whether Clostridum botulinum type A toxin-haemagglutinin complex is excreted in human milk. The excretion of Clostridum botulinum type A toxin-haemagglutinin complex in milk has not been studied in animals. The use of Clostridum botulinum type A toxin-haemagglutinin complex during lactation cannot be recommended.
4.7 Effects on ability to drive and use machines
There is a potential risk of muscle weakness or visual disturbances which, if experienced, may temporarily impair the ability to drive or operate machinery.
4.8 Undesirable effects
Very common >1/10: Common >1/100, <1/10: Uncommon >1/1000, <1/100:
Rare >1/10000, < 1/1000: Very rare <1/10000.
Side effects related to spread of toxin distant from the site of administration have been reported (exaggerated muscle weakness, dysphagia, aspiration/aspiration pneumonia, with fatal outcome in some very rare cases) (see section 4.4).
General
In the clinical trial programme, approximately 28% of the patients treated with Dysport experienced an adverse event.
The following adverse reactions were seen in patients treated across a variety of indications including blepharospasm, hemifacial spasm, torticollis and spasticity associated with either cerebral palsy or stroke:
Nervous system disorders
Rare: Neuralgic amyotrophy
Skin and subcutaneous tissue disorders
Uncommon: Pruritus
Rare: Rash
General disorders and administration site conditions
Common: Asthenia, fatigue, influenza like illness, injection site pain/bruising
In addition, the following adverse reactions specific to individual indications were reported:
Arm spasticity
Gastrointestinal disorders
Common: Dysphagia
Musculoskeletal and connective tissue disorders Common: Arm muscle weakness Injury, poisoning and procedural complications Common: Accidental injury/fall
Paediatric cerebral palsy spasticity
Gastrointestinal disorders
Common: Diarrhoea
Musculoskeletal and connective tissue disorders
Common: Leg muscle weakness, muscle pain Renal and urinary disorders
Common: Urinary incontinence General disorders and administration site conditions Common: Abnormal gait Injury, poisoning and procedural complications Common: Accidental injury/fall
Accidental injury due to falling and abnormal gait may have been due to the overweakening of the target muscle and / or the local spread of Dysport to other muscles involved in ambulation and balance.
Spasmodic torticollis
Nervous system disorders
Common: Headache, dizziness, facial pareis
Eye disorders
Common: Vision blurred, visual acuity reduced Uncommon: Diplopia, ptosis
Respiratory, thoracic and mediastinal disorders Common: Dysphonia, dyspnoea Rare: Aspiration Gastrointestinal disorders
Very common: Dysphagia, dry mouth Uncommon: Nausea
Musculoskeletal and connective tissue disorders Very Common: Muscle weakness
Common: Neck pain, musculoskeletal pain, myalgia, pain in extremity
musculoskeletal stiffness
Uncommon: Muscle atrophy, jaw disorder
Dysphagia appeared to be dose related and occurred most frequently following injection into the sternomastoid muscle. A soft diet may be required until symptoms resolve.
These side effects may be expected to resolve within two to four weeks.
Blepharospasm and hemifacial spasm
Nervous system disorders
Common: Facial paresis Uncommon: VIIth nerve paralysis Eye disorders
Very common: Ptosis
Common: Diplopia, dry eye, lacrimation increased Rare: Ophthalmoplegia
Skin and subcutaneous tissue disorders Common: Eyelid oedema Rare: Entropion
Side effects may occur due to deep or misplaced injections of Dysport temporarily paralysing other nearby muscle groups.
Post-marketing experience
The profile of adverse reactions reported to the company during postmarketing use reflects the pharmacology of the product and those seen during clinical trials. In addition, hypersensitivity reactions have been reported.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
4.9. Overdose
Excessive doses may produce distant and profound neuromuscular paralysis. Overdose could lead to an increased risk of the neurotoxin entering the bloodstream and may cause complications associated with the effects of oral botulinum poisoning (e.g. dysphagia and dysphonia). Respiratory support may be required where excessive doses cause paralysis of respiratory muscles. There is no specific antidote; antitoxin should not be expected to be beneficial and general supportive care is advised. In the event of overdose the patient should be medically monitored for signs and/or symptoms of excessive muscle weakness or muscle paralysis. Symptomatic treatment should be instigated if necessary.
Symptoms of overdose may not present immediately following injection. Should accidental injection or oral ingestion occur, the patient should be medically supervised for several weeks for signs and/or symptoms of excessive muscle weakness or muscle paralysis.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic Group: Other muscle relaxants, peripherally acting agents.
ATC code: M03AX01
Clostridium botulinum type A toxin-haemagglutinin complex blocks peripheral cholinergic transmission at the neuromuscular junction by a presynaptic action at a site proximal to the release of acetylcholine. The toxin acts within the nerve ending to antagonise those events that are triggered by Ca2+ which culminate in transmitter release. It does not affect postganglionic cholinergic transmission or postganglionic sympathetic transmission.
The action of toxin involves an initial binding step whereby the toxin attaches rapidly and avidly to the presynaptic nerve membrane. Secondly, there is an internalisation step in which toxin crosses the presynaptic membrane, without causing onset of paralysis. Finally the toxin inhibits the release of acetylcholine by disrupting the Ca2+ mediated acetylcholine release mechanism, thereby diminishing the endplate potential and causing paralysis.
Recovery of impulse transmission occurs gradually as new nerve terminals sprout and contact is made with the postsynaptic motor endplate, a process which takes 6 - 8 weeks in the experimental animal.
Focal spasticity affecting the upper limbs
The efficacy and safety of Dysport for the treatment of upper limb spasticity was evaluated in a randomized, multi-centre, double-blind, placebo-controlled study that included 238 patients (159 Dysport and 79 placebo) with upper limb spasticity who were at least 6 months post-stroke (90%) or post-traumatic brain injury (10%). The primary targeted muscle group (PTMG) was the extrinsic finger flexors (56%), followed by the elbow (28%) and wrist flexors (16%).
The primary efficacy variable was the PTMG muscle tone at week 4, as measured by the Modified Ashworth Scale (MAS), a 5 point scale ranging from 0 (no increase in muscle tone) to 4 (affected in part[s] rigid in flexion or extension) and the first secondary endpoint was the Physician Global Assessment (PGA) of response to treatment (a 9 point scale ranging from -4 [markedly worse], through 0 [no change], to +4 [markedly improved]). The main results achieved at Week 4 and Week 12 are shown below:
|
Week 4 |
Week 12 | |||||
|
Placebo (N=79) |
Dysport (500 units) (N=80) |
Dysport (1000 units) (N=79) |
Placebo (N=79) |
Dysport (500 units) (N=80) |
Dysport (1000 units) (N=79) | |
|
LS Mean Change from Baseline in PTMG Muscle Tone on the MAS |
-0.3 |
-1.2** |
-1 4** |
-0.1 n=75 |
-0 7** n=76 |
-0.8** n=76 |
|
LS Mean PGA of Response to Treatment |
0.7 |
1.4* |
1.8** |
0.4 n=75 |
0.5 n=76 |
1.0* n=76 |
|
LS Mean Change from Baseline in Wrist Flexor Muscle Tone on the MAS |
-0.3 n=54 |
-1 4** n=57 |
-1.6** n=58 |
-0.3 n=52 |
-0.7* n=54 |
-0.9* n=56 |
|
LS Mean Change from Baseline in Finger Flexor Muscle Tone on the MAS |
-0.3 n=70 |
-0.9* n=66 |
-1.2** n=73 |
-0.1 n=67 |
-0.4* n=62 |
-0.6* n=70 |
|
LS Mean Change from Baseline in Elbow Flexor Muscle Tone on the MAS |
-0.3 n=56 |
-1.0* n=61 |
-1.2** n=48 |
-0.3 n=53 |
-0.7* n=58 |
-0.8* n=46 |
|
Mean Change from Baseline in Shoulder Extensors Muscle Tone on the MAS (1) |
-0.4 n=12 |
-0.6 n=7 |
-0.7 n=6 |
0.0 n=12 |
-0.9 n=7 |
0.0 n=6 |
|
*p<0.05; ** p<0.000 LS = Least Square (1) No statistical tests groups as there are lim |
5 performed due to low frequency by treatment and placebo nited data in patients treated in the shoulder muscles. | |||||
The Principal Target of Treatment (PTT) of the Disability Assessment Scale [DAS] was used to investigate the effect of treatment on functional impairment (passive function). Although some improvement in the mean change from baseline at Week 4 in the Dysport groups did not reach statistical significance compared to placebo, the proportion of DAS score responders (subjects achieving at least a one grade improvement) for the PTT was significantly higher at the 1000U dose as shown below.
|
Treatment Group |
Week 4 |
Week 12 |
|
% Responders |
% Responders | |
|
Dysport 500U |
50.0 |
41.3 |
|
n=80 |
n=76 | |
|
p = 0.13 |
p = 0.11 | |
|
Dysport 1000U |
62.0 |
55.7 |
|
n=78 |
n=76 | |
|
p = 0.0018 |
p = 0.0004 | |
|
Placebo |
39.2 |
32.9 |
n=79
n=75
*Domains included in DAS are hygiene, limb position, dressing and pain.
In addition, statistically significant improvements in spasticity (grade and angle) assessed by the Tardieu scale, in the active range of motion of the fingers, wrist or elbow, and in ease of applying a splint by the subject were observed, especially at the 1000U dose. However, there was no effect of treatment shown on the active function, as assessed by the Modified Frenchay Score, and on quality of life EQ5D or SF-36 questionnaires.
Blepharospasm
Three Dysport doses were investigated over 1 treatment cycle in a clinical study.
Efficacy was measured by the medians of differences in the Percentage of Normal Activity (PNA) values (derived from the Blepharospasm Disability Scale) between each treatment group and placebo. A dose-dependent improvement in blepharospasm was evident with increasing Dysport dose, with all treatment groups being superior to placebo.
|
Difference between the median of the changes in PNA values from baseline in the active group and the median of the changes in PNA values from baseline in the placebo group Visit |
Dysport 40 Units (N=30) |
Dysport 80 Units (N=31) |
Dysport 120 Units (N=31) |
|
Week 4: |
31.2 % |
41.3 % |
48.5 % |
|
Week 8: |
36.0 % |
48.3 % |
55.0 % |
|
Week 12: |
36.0 % |
36.3 % |
50.0 % |
|
Week 16: |
10.5 %[a] |
24.2 % |
31.3 % |
[a] p value > 0.001
For the 40 units, 80 units and 120 units Dysport treatment groups, the medians of the changes from baseline in PNA values were statistically significantly higher compared to those in placebo group at weeks 4, 8, and 12.
A statistically significant difference compared to placebo group was also observed for the 80 units and 120 units Dysport treatment groups at week 16, indicating a greater duration of response at the 80 units and 120 units doses.
The incidence of related Treatment Emergent Adverse Events (TEAEs), specifically ptosis, was higher in the Dysport treatment groups than in the placebo treatment group and was dose-dependent with greater incidence seen at higher Dysport doses. See table below.
|
Statistic |
Placebo (N=26) |
Dysport 40 Units (N=31) |
Dysport 80 Units (N=31) |
Dysport 120 Units (N=31) | |
|
Patients with related TEAEs |
n (%) |
3 (12) |
19 (61) |
23 (74) |
26 (84) |
|
Patients with | |||||
|
related eye TEAEs |
n (%) |
3 (12) |
16 (52) |
23 (74) |
26 (84) |
5.2 Pharmacokinetic properties
Pharmacokinetic studies with botulinum toxin pose problems in animals because of the high potency, the minute doses involved, the large molecular weight of the compound and the difficulty of labelling toxin to produce sufficiently high specific activity. Studies using I125 labelled toxin have shown that the receptor binding is specific and saturable, and the high density of toxin receptors is a contributory factor to the high potency. Dose and time responses in monkeys showed that at low doses there was a delay of 2-3 days with peak effect seen 5-6 days after injection. The duration of action, measured by changes of ocular alignment and muscle paralysis varied between 2 weeks and 8 months. This pattern is also seen in man, and is attributed to the process of binding, internalisation and changes at the neuromuscular junction.
5.3 Preclinical safety data
Reproductive toxicity studies in pregnant rats and rabbits given Clostridium botulinum type A toxin-haemagglutinin complex by daily intramuscular injection, at doses of 6.6 units/kg (79 units/kg total cumulative dose) and 3.0 units/kg (42 units/kg total cumulative dose) in rats and rabbits respectively, did not result in embryo/foetal toxicity. Implantation losses at maternally toxic doses were observed at higher doses in both species. Clostridium botulinum type A toxin-haemagglutinin complex demonstrated no teratogenic activity in either rats or rabbits and no effects were observed in the pre- and postnatal study on the F1 generation in rats. Fertility of male and female rats was decreased due to reduced mating secondary to muscle paralysis at doses of
29.4 units/kg weekly in males and increased implantation loss at 20 units/kg weekly in females.
In a chronic toxicity study performed in rats up to 12 units/animal, there was no indication of systemic toxicity. Effects in chronic toxicity non-clinical studies were limited to changes on injected muscles related to the mechanism of action of Clostridium botulinum type A toxin-haemagglutinin complex. There was no ocular irritation following administration of Clostridium botulinum type A toxin-haemagglutinin complex into the eyes of rabbits.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Human albumin solution
Lactose
6.2 Incompatibilities
None known.
6.3 Shelf life
Unopened vial:
24 months
Reconstituted solution:
Chemical and physical in-use stability has been demonstrated for 24 hours at 2°C-8°C.
From a microbiological point of view, unless the method of reconstitution precludes the risk of microbial contamination, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user.
6.4 Special precautions for storage Unopened vial:
Store in a refrigerator (2°C-8°C).
Do not freeze.
Reconstituted solution:
For storage conditions after reconstitution of the medicinal product, see section 6.3.
6.5 Nature and contents of container Nature of container/closure:
Type 1 glass vials with 3 mL capacity. 13 mm bromobutyl freeze-drying closures oversealed by 13 mm aluminium overseals with centre hole, crimped over.
Contents of container:
A white lyophilised powder for reconstitution.
6.6 Special precautions for disposal
The exposed central portion of the rubber stopper should be cleaned with alcohol immediately prior to piercing the septum. A sterile 23 or 25 gauge needle should be used.
Each vial is for single use only,
Reconstitution instructions are specific for each of the 300 Unit vial and the 500 Unit vial. These volumes yield concentrations specific for the use for each indication.
|
Resulting Dose Unit per mL |
Diluent* per 500 Unit Vial |
Diluent* per 300 Unit Vial |
|
500 Units |
1 mL |
0.6 mL |
|
200 Units |
2.5 mL |
1.5 mL |
|
100 Units |
5 mL |
3 mL |
*Preservative-free 0.9% sodium chloride injection
Immediately after treatment of the patient, any residual Dysport which may be present in either vial or syringe should be inactivated with dilute hypochlorite solution (1% available chlorine).
Spillage of Dysport should be wiped up with an absorbent cloth soaked in dilute hypochlorite solution.
Any unused product or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
Ipsen Limited 190 Bath Road Slough Berkshire SL1 3XE United Kingdom
8 MARKETING AUTHORISATION NUMBER(S)
PL 34926/0001
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 09 December 1990 Date of latest renewal: 17 December 2002
10 DATE OF REVISION OF THE TEXT
14/06/2016