Eigenorm Pfs 150 Iu Powder And Solvent For Solution For Injection
Eigenorm PFS 75IU-150IU
Powder and solvent for solution for injection
1.3.1
PACKAGE LEAFLET
Eigenorm PFS 75 IU
powder and solvent for solution for injection
Eigenorm PFS 150 IU
powder and solvent for solution for injection
Menotrophin
▼ This medicine is subject to additional monitoring. This will allow quick identification of new safety information. You can help by reporting any side effects you may get. See the end of section 4 for how to report side effects.
Read all of this leaflet carefully before you start using this medicine because it contains
important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, please ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
• In this leaflet Eigenorm PFS 75 IU powder and solvent for solution for injection and Eigenorm PFS 150 IU powder and solvent for solution for injection are called Eigenorm PFS.
What is in this leaflet:
1. What Eigenorm PFS is and what it is used for
2. What you need to know before you use Eigenorm PFS
3. How to use Eigenorm PFS
4. Possible side-effects
5. How to store Eigenorm PFS
6. Contents of the pack and other information
1. What Eigenorm PFS is and what it is used for
• Eigenorm PFS is used to promote ovulation in women who are not ovulating and who have not responded to other treatment (clomiphene citrate).
• Eigenorm PFS is used to bring about the development of several follicles (and therefore several eggs) in women receiving fertility treatment.
Eigenorm PFS is a highly purified human menopausal gonadotrophin, belonging to a group of medicines called gonadotrophins.
Each freeze-dried vial contains 75 IU human follicle stimulating hormone activity (FSH) and 75 IU human luteinising hormone activity (LH).
Human Chorionic Gonadotrophin (hCG), a hormone naturally present in urine of pregnant women, is added to contribute to the total LH activity.
Each freeze-dried vial contains 150 IU human follicle stimulating hormone activity (FSH) and 150 IU human luteinising hormone activity (LH).
Human Chorionic Gonadotrophin (hCG), a hormone naturally present in urine of pregnant women, is added to contribute to the total LH activity.
This medicinal product must be used under the supervision of your doctor.
2. What you need to know before you use Eigenorm PFS
You and your partner’s fertility will be evaluated before your treatment is started.
Do not use Eigenorm PFS if you have any of the following:
• Enlarged ovaries or cysts not caused by a hormonal disorder (polycystic ovarian disease).
• Bleeding of unknown cause.
• Cancer of the ovaries, uterus or breast.
• Abnormal swelling (tumour) of the pituitary gland or hypothalamus (brain).
• Hypersensitivity (allergy) to Menotrophin or any of the ingredients in Eigenorm PFS
This medicine should not be used if you have an early menopause, a malformation of the sexual organs or certain tumours of the womb that would make a normal pregnancy impossible.
Warnings and Precautions
Although no allergic reactions to Eigenorm PFS have yet been reported, you should tell your doctor if you have an allergic reaction to similar medicines.
This treatment increases your risk of developing a condition known as ovarian hyperstimulation syndrome (OHSS) (see Possible side effects). If ovarian hyperstimulation occurs then your treatment will be stopped and pregnancy will be avoided. The first signs of ovarian hyperstimulation are pain in the lower abdominal region as well as nausea (feeling sick), vomiting and weight gain. If these symptoms occur you should be examined by your doctor as soon as possible. In serious, but rare cases, the ovaries can become enlarged and fluid can build up in the abdomen or chest.
The drug used to bring about the final release of mature eggs (containing human chorionic gonadotrophin-hCG) can increase the likelihood of OHSS. It is therefore not advisable to use hCG in cases where OHSS is developing and you should not have sexual intercourse even if using a barrier method of contraception for at least 4 days.
It should be noted that women with fertility problems have a higher rate of miscarriages than the normal population.
In patients having treatment to help ovulation, the occurrence of multiple pregnancies and births is increased compared to natural conception. However, this risk can be minimised by using the recommended dose.
There is a slightly increased risk of extra-uterine pregnancy (an ectopic pregnancy) in women with damaged fallopian tubes.
Multiple pregnancies and characteristics of the parents undergoing fertility treatments (e.g. maternal age, sperm characteristics) may be associated with an increased risk of birth defects.
Treatment with Eigenorm PFS, just as pregnancy itself, may increase the chance of having thrombosis. Thrombosis is the formation of a blood clot in a blood vessel, most often in the veins of the legs or the lungs.
Please discuss this with your doctor, before starting treatment, especially:
• If you already know you have an increased chance of having thrombosis.
• If you, or anyone in your immediate family, have ever had a thrombosis.
• If you are severely overweight.
Paediatric population
The product is not intended for paediatric use.
Other medicines and Eigenorm PFS
Tell your doctor or pharmacist if you are taking or have recently taken or might take any other medicines.
Pregnancy, breast-feeding and fertility
Eigenorm PFS should not be used if you are pregnant or breast-feeding.
Driving and using machines
Eigenorm PFS has no or negligible influence on the ability to drive and use of machinery. However, no studies on the effect on ability to drive and use machines have been performed.
3. How to use Eigenorm PFS
Dosage and duration of the treatment:
Always use this medicine exactly as your doctor has told you. Check with your doctor if you are not sure.
Women who are not ovulating and are having irregular periods or no periods at all:
As a general rule, the first injection of one Eigenorm PFS 75 IU vial is given during the first week of the cycle after spontaneous or induced menses.
Subsequently, Eigenorm PFS is injected daily at the dosage prescribed by the physician and the treatment will continue until one or more ripe follicle have developed in the ovary. Your physician will adjust the Eigenorm PFS dosage depending on the ovarian response, which is determined by clinical examinations.
As soon as one follicle reaches the required development stage, the Eigenorm PFS treatment will be withheld and ovulation will be triggered with another hormone (chorionic gonadotropin, hCG).
Ovulation generally takes place after 32 to 48 hours.
In this phase of the treatment, fertilization is possible. You will be advised to have sexual intercourse every day starting from the day preceding the administration of hCG. If pregnancy is not achieved in spite of ovulation, the treatment can be repeated.
Women undergoing ovarian stimulation for multiple follicular development prior to in vitro fertilisation or other assisted reproductive techniques:
The aim of this method is to obtain concomitant multiple follicular development. The treatment will start on the 2nd or 3rd day of the cycle with injections of 150-300 IU of Eigenorm PFS (1-2 vials of Eigenorm PFS 150 IU). Your physician may decide to administer higher dosages if required. The injected dosage of Eigenorm PFS is higher than in the method used for natural fertilization. The continuation of the treatment is adjusted individually by the physician.
As soon as a sufficient number of follicles has developed, the treatment with Eigenorm PFS is withheld and ovulation is triggered by injecting another hormone (chorionic gonadotropin, hCG).
How Eigenorm PFS should be given:
Eigenorm PFS is given by injection under your skin (by the subcutaneous route) or into your muscle (intramuscular injection).
Each vial should be used only once and the injection should be used as soon as it is prepared.
After suitable advice and training your doctor may ask you to inject Eigenorm PFS yourself.
For the first time, your doctor must:
- let you practise giving yourself a subcutaneous injection,
- have shown you the possible places where you can inject yourself,
- have shown you how to prepare the solution for injection,
- have explained how to prepare the right dose of injection.
Before injecting Eigenorm PFS yourself, read the following instructions carefully.
How to prepare and inject 1 vial of Eigenorm PFS:
The injection must be prepared just before you are ready to use it, using the prefilled syringe of solvent (a solution of 0.9% sodium chloride in water for injections) included in each Eigenorm PFS pack.
Prepare a clean surface and wash your hands. It is important that your hands and the items you use are as clean as possible.
On the surface lay out the following items:
- two alcohol swabs (not provided),
- one vial containing Eigenorm PFS powder,
- one prefilled syringe with solvent,
- one needle for preparing the injection,
- a fine bore needle for subcutaneous injection.
Reconstitution of the solution for injection Preparing your injection:
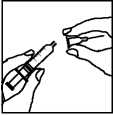
1. • Remove the cap from the prefilled syringe; insert the
reconstitution needle (long needle) on the syringe.
• Carefully place the syringe on the clean surface.
• Avoid touching the needle.
Prepare the solution for the injection:
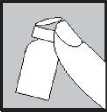
• Remove the coloured plastic cap (75 IU light green,
3.
150 IU dark green) from the Eigenorm PFS vial by gently pushing it upwards.
• Wipe the rubber top with an alcohol swab and allow to dry.
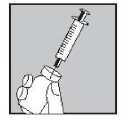
• Pick up the syringe, remove the protective cap of the needle and push the needle through the rubber middle of the Eigenorm PFS vial top.
• Press the plunger down firmly to squirt all the solution onto the powder.
• DO NOT SHAKE, but gently swirl until the solution is clear.
Generally Eigenorm PFS dissolves immediately.
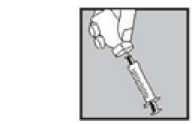
4. • With the needle still inserted, turn the vial upside down.
• Make sure the needle tip is underneath the level of the liquid.
• Gently pull the plunger to draw all the Eigenorm PFS solution up into the syringe.
• Check that the reconstituted solution is clear.
When reconstituting more than 1 vial of Eigenorm PFS, draw back the reconstituted contents of the first vial into the syringe and slowly inject into a second vial after repeating the steps
2-4.
Injecting your medicine subcutaneously:
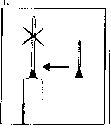
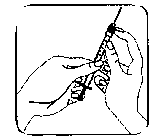
The injection site:
When the syringe contains the described dose, attach the protective cap of the needle. Remove the needle from the syringe and replace it with the fine bore needle for subcutaneous injection including its protective cap.
Push the fine bore needle firmly onto the syringe barrel, then twist it slightly to ensure it is fully screwed on and to create a firm seal. Remove the protective cap of the needle. Hold the syringe with the needle pointing upwards and gently tap the side of the syringe to force any air bubbles up to the top;
Push the plunger until a bead of liquid appears at the tip of the needle. Do not use if it contains any particles or is cloudy.
Your doctor or nurse will have already advised you where on your body to inject your medicine. The usual places are the thigh or the lower abdominal wall below the navel.
Wipe the injection site with an alcohol swab.
Inserting the needle:
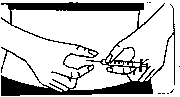
• Firmly pinch the skin together. With the other hand, insert the needle with a dart-like motion at an angle of 45° or 90°.
Injecting the solution:
• Inject under the skin as you were shown. Do not inject directly into a vein. Push the plunger slowly and steadily, so the solution is correctly injected and the skin tissues are not damaged.
Take as much time as you need to inject the volume of solution prescribed. Depending on the dosage prescribed by your doctor, you may not use the entire volume of the solution.
Removing the needle:
• Pull the syringe out quickly and apply pressure to the injection site with a swab containing disinfectant. A gentle massage of the site - while still maintaining pressure - helps disperse the Eigenorm PFS solution and relieve any discomfort.
Injecting your medicine intramuscularly:
For intramuscular injections your healthcare provider will prepare and then inject Eigenorm PFS into the side of your thigh or buttock.
Dispose of all used items:
Once you have finished your injection, put all needles and empty vials and syringes into the sharps container. Any unused solution or waste material should be disposed of in accordance with local requirements.
If you use more Eigenorm PFS than you should:
The effects of an overdose of Eigenorm PFS are unknown, nevertheless, one could expect ovarian hyperstimulation syndrome to occur (see Possible side effects). If you use more Eigenorm PFS than you should, speak to your doctor or pharmacist.
If you forget to use Eigenorm PFS:
Take it at the next normal time for an injection. Do not take double dose to make up for a forgotten dose.
If you stop using Eigenorm PFS:
Do not stop on your own initiative: Always consult your doctor if you are considering stopping this medicine.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Eigenorm PFS can cause side effects, although not everybody gets them.
The following side effect is important and will require immediate action if you experience it.
You should stop taking Eigenorm PFS and see your doctor immediately if the following
occurs:
Common, affects 1 to 10 users in 100:
• Ovarian Hyperstimulation Syndrome (symptoms include ovarian cyst formation or enlargement of existing cysts, lower stomach pain, feeling thirsty and sick, and sometimes being sick, passing reduced quantities of concentrated urine and weight gain) (see Section 2 for additional information).
The following side-effects have also been reported:
Very Common (likely to affect up to 1 in 10 users):
• Headache
• Swollen or bloated stomach
Common (likely to affect between 1 in 100 and 1 in 10 users):
• Abdominal pain or discomfort
• Pelvic pain
• Back pain
• Sensation of heaviness
• Breast discomfort
• Dizziness
• Hot flushes
• Thirst
• Feeling sick
• Tiredness
• Feeling generally unwell
• Injection site reaction such as pain and inflammation (frequency higher with IM than SC).
Rare, (likely to affect between 1 in 10,000 and 1 in 1000 users):
• Ovarian torsion (twisting of the ovary which causes extreme pain in the lower abdomen)
Very rare, (likely to affect between 1 in 100,000 and 1 in 10,000 users):
• Thromboembolism (formation of a clot in a blood vessel that breaks loose and is carried by the blood stream to block another vessel).
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Eigenorm PFS
Keep this medicine out of the sight and reach of children.
Do not store above 25° C. Keep the vial and the prefilled syringe of solvent in the outer carton in order to protect from light.
Do not use this medicine after the expiry date which is stated on the outer carton, the vial and the prefilled syringe of solvent. The expiry date refers to the last day of the month.
Use immediately after reconstitution.
Do not use Eigenorm PFS if you notice the solution does not look clear. After reconstitution the solution must be clear and colourless.
Do not throw away any medicines via wastewater. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Eigenorm PFS contains The active substance is menotrophin.
Each freeze-dried vial contains 75 IU human follicle stimulating hormone activity (FSH) and 75 IU human luteinising hormone activity (LH).
Human Chorionic Gonadotrophin (hCG), an hormone naturally present in urine of pregnant women, is added to contribute to the total LH activity.
Each freeze-dried vial contains 150 IU human follicle stimulating hormone activity (FSH) and 150 IU human luteinising hormone activity (LH).
Human Chorionic Gonadotrophin (hCG), an hormone naturally present in urine of pregnant women, is added to contribute to the total LH activity.
If multiple vials of powder are used, the amount of menotrophin contained in 1 ml of reconstituted solution will be as follows:
|
Eigenorm PFS 75 IU powder and solvent for solution for injection | |
|
Number of vials used |
Total amount of menotrophin in 1 ml of solution |
|
1 |
75 IU |
|
2 |
150 IU |
|
3 |
225 IU |
|
4 |
300 IU |
|
5 |
375 IU |
|
6 |
450 IU |
|
Eigenorm PFS 150 IU powder and solvent for solution for injection | |
|
Number of vials used |
Total amount of menotrophin in 1 ml of solution |
|
1 |
150 IU |
|
2 |
300 IU |
|
3 |
450 IU |
The other excipients are
For the powder: lactose monohydrate.
For the solvent: 0.9% sodium chloride solution.
What Eigenorm PFS looks like and contents of the pack
Powder: white to almost white lyophilized powder Solvent: clear and colourless solution
Eigenorm PFS is presented as a powder and solvent for solution for injection.
1 set contains the following:
• One vial containing a white to almost white powder
• One prefilled syringe (1ml) containing a clear and colourless solution
• One needle for reconstitution and intramuscular injection (long needle)
• One needle for subcutaneous injection (short needle)
It comes in pack sizes of 1, 5 or 10 sets. Not all pack sizes may be marketed.
Marketing Authorisation Holder:
IBSA Farmaceutici Italia srl Via Martiri di Cefalonia, 2 26900 Lodi - Italy
Manufacturer:
IBSA Farmaceutici Italia srl Via Martiri di Cefalonia, 2 26900 Lodi - Italy
This medicinal product is authorized in the Member States of the EEA under the following names: (The strength and pharmaceutical form are identical in all countries, only the trade name changes)
Austria: Meriofert PFS Belgium: Fertinorm Kit Bulgaria: Meriofert PFS Cyprus: Meriofert PFS Czech Republic: Eigenorm Set Denmark: Meriofert Set France: Fertistartkit Greece: Meriofert Hungary: Meriofert Kit Italy: Meriofert Luxembourg: Fertinorm Kit Poland: Mensinorm Romania: Meriofert PFS Slovakia: Meriofert Spain: Meriofert Kit The Netherlands: Meriofert spuit United Kingdom: Eigenorm PFS
This leaflet was last revised November 2015.
Ed. 2015-001 12