Elashine 104 Mg Suspension For Injection In Pre-Filled Syringe
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
ELASHINE 104 mg suspension for injection in pre-filled syringe
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
ELASHINE single dose pre-filled syringe containing 104 mg medroxyprogesterone acetate (MPA) in 0.65 ml suspension for injection.
Excipients with known effect
Each pre-filled syringe contains:
Methyl parahydroxybenzoate (E218) - 1.04 mg per 0.65ml Propyl parahydroxybenzoate (E216) - 0.0975 mg per 0.65ml Sodium - 2.47 mg per 0.65ml
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Suspension for injection in pre-filled syringe White to off-white homogeneous suspension
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
ELASHINE is indicated for long-term female contraception. Each subcutaneous injection prevents ovulation and provides contraception for at least 13 weeks (+/- 1 week). However, it should be taken into consideration that the return to fertility (ovulation) may be delayed for up to one year (see section 4.4).
Since loss of bone mineral density (BMD) may occur in females of all ages who use ELASHINE long-term (see section 4.4), a risk/benefit assessment, which also takes into consideration the decrease in BMD that occurs during pregnancy and/or lactation, should be performed before administration of ELASHINE.
It is also important that the patient is informed about the long-term nature of this product’s effects, including a delayed return to fertility (see section 4.4).
Use in Adolescents (12-18 years)
In adolescents, use of ELASHINE is only indicated when other contraceptive methods are considered unsuitable or unacceptable, due to unknown long-term effects of bone loss associated with ELASHINE during the critical period of bone accretion (see section 4.4)
ELASHINE has not been studied in women under the age of 18 years but data is available for intramuscular MPA in this population.
4.2 Posology and method of administration
The pre-filled syringe of ELASHINE should be vigorously shaken just before use to ensure that the dose being given represents a uniform suspension. The treatment should be initiated by a doctor or healthcare assistant and administered as a subcutaneous injection (SC) into the anterior thigh or abdomen. The medication should be injected slowly until the syringe is empty, which should take about 5-7 seconds. For instructions on how to prepare ELASHINE before administration, see section 6.6.
Step 1: Choosing and preparing the injection area.
Choose the injection area in either the upper thigh or abdomen, see shaded areas (Diagram 1). Avoid bony areas and the umbilicus.
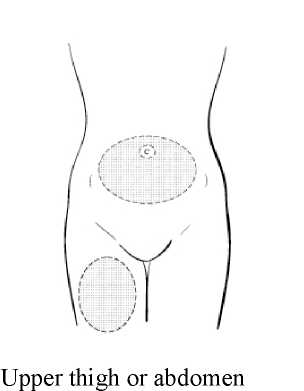
Diagram 1
Use an alcohol pad to wipe the skin in the injection area you have chosen. Allow the skin to dry.
While continuing to hold the syringe barrel firmly, remove the protective plastic needle cover from the needle without twisting, making sure the needle is still firmly attached to the syringe (Diagram 2).
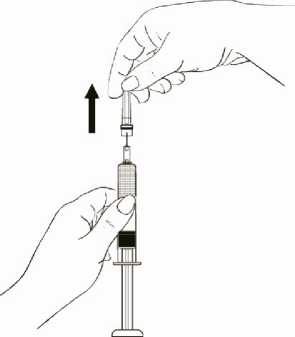
Diagram 2
While holding the syringe with the needle pointing upward, gently push in the plunger until the medicine is up to the top of the syringe. There should be no air within the barrel (Diagram 3).
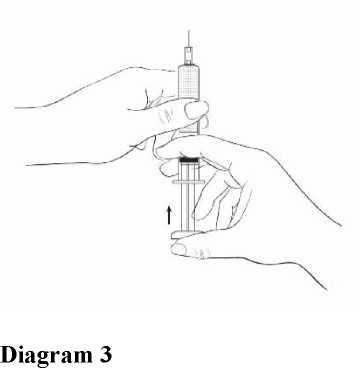
Step 2: Injecting the dose.
Gently grasp and squeeze a large area of skin in the chosen injection area between the thumb and fore-finger, pulling it away from the body. Insert the needle at a 45 degree angle so that most of the needle is in the fatty tissue. The plastic hub of the needle should be nearly or almost touching the skin (Diagram 4).
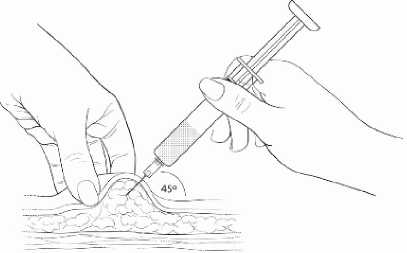
Diagram 4
Inject the medication slowly until the syringe is empty (Diagram 5).
• This should take about 5-7 seconds.
• It is important that the entire dose of ELASHINE is given
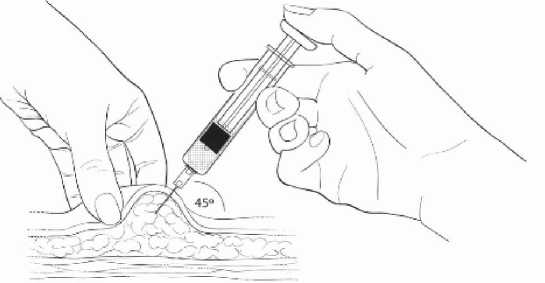
Inject slowly (5-7 seconds)
Diagram 5
When the entire dose is completely injected, gently pull the needle out of the skin.
Use a clean cotton pad to press lightly on the injection area for a few seconds. Do NOT rub the area.
Adults
First Injection: To provide contraceptive cover in the first cycle of use, an injection of 104 mg SC should be given during the first five days of a normal menstrual cycle. If the injection is carried out according to these instructions, no additional contraceptive measure is required.
Further doses: The second and subsequent injections should be given at 13 week intervals, as long as the injection is given no later than seven days after this time, no additional contraceptive measures (e.g. barrier) are required. If the interval from the preceding injection is greater than 14 weeks (13 weeks plus 7 days) for any reason, then pregnancy should be excluded before the next injection is given. The efficacy of ELASHINE depends on adherence to the recommended dosage schedule of administration.
PostPartum: If the patient is not breast-feeding, the injection should be given within 5 days post partum (to increase assurance that the patient is not pregnant). If the injection is to be given at another time then pregnancy should be excluded.
If the patient is breast-feeding, the injection should be given no sooner than six weeks post partum, when the infant's enzyme system is more developed (see section 4.6).
There is evidence that women prescribed ELASHINE in the immediate puerperium can experience prolonged and heavy bleeding. Because of this, the drug should be used with caution in the puerperium. Women who are considering use of the product immediately following delivery or termination should be advised that the risk of heavy or prolonged bleeding may be increased. Doctors are reminded that in the non breast-feeding, post partum patient, ovulation may occur as early as week 4.
Switching from other Methods of Contraception: When switching from other contraception methods, ELASHINE should be given in a manner that ensures continuous contraceptive coverage based upon the mechanism of action of both methods, (e.g. patients switching from oral contraceptives should have their first injection of ELASHINE within 7 days after their last active pill).
Special populations
Patients with hepatic impairment: The effect of hepatic disease on the pharmacokinetics of ELASHINE is unknown. As ELASHINE largely undergoes hepatic elimination it may be poorly metabolised in patients with severe liver insufficiency (see section 4.3).
Patients with renal impairment: The effect of renal disease on the pharmacokinetics of ELASHINE is unknown. No dosage adjustment should be necessary in women with renal insufficiency, since ELASHINE is almost exclusively eliminated by hepatic metabolism.
Paediatric population
ELASHINE is not indicated before menarche (see section 4.1). Data in adolescent females (12-18 years) is available for IM administration of MPA (see section 4.4 and section 5.1). Other than concerns about loss of BMD, the safety and effectiveness of ELASHINE is expected to be the same for adolescents after menarche and adult females
4.3 Contraindications
• Medroxyprogesterone acetate is contra-indicated in patients with a known hypersensitivity to MPA or any of its excipients listed in section 6.1.
• if pregnancy is known or suspected.
• in women with known or suspected malignancy of the breast or genital organs.
• in patients with undiagnosed vaginal bleeding.
• in patients with severe hepatic impairment.
• in patients with metabolic bone disease.
• in patients with active thromboembolic disease and in patients with current or past history of cerebrovascular disease.
4.4 Special warnings and precautions for use
Loss of Bone Mineral Density:
Use of ELASHINE reduces serum estrogen levels and is associated with significant loss of BMD due to the known effect of estrogen deficiency on the bone remodelling system. Bone loss is greater with increasing duration of use, however BMD appears to increase after ELASHINE is discontinued and ovarian estrogen production increases.
This loss of BMD is of particular concern during adolescence and early adulthood, a critical period of bone accretion. It is unknown if use of ELASHINE by younger women will reduce peak bone mass and increase the risk for fracture in later life.
A study to assess the BMD effects of medroxyprogesterone acetate IM (Depo-Provera, DMPA) in adolescent females showed that its use was associated with a significant decline in BMD from baseline. In the small number of women who were followed-up, mean BMD recovered to around baseline values by 1- 3 years after discontinuing treatment. In adolescents, ELASHINE may be used, but only after other methods of contraception have been discussed with the patients and considered to be unsuitable or unacceptable.
In women of all ages, careful re-evaluation of the risks and benefits of treatment should be carried out in those who wish to continue use for more than 2 years. In particular, in women with significant lifestyle and/or medical risk factors for osteoporosis, other methods of contraception should be considered prior to use of ELASHINE.
Significant risk factors for osteoporosis include:
• Alcohol abuse and/or tobacco use
• Chronic use of drugs that can reduce bone mass, e.g., anticonvulsants or
corticosteroids
• Low body mass index or eating disorder, e.g., anorexia nervosa or bulimia
Previous low trauma fracture Family history of osteoporosis
A retrospective cohort study using data from the General Practice Research Database (GPRD) reported that women using MPA injections (DMPA), have a higher risk of fracture compared with contraceptive users with no recorded use of DMPA (incident rate ratio 1.41, 95% CI 1.35-1.47 for the five year follow-up period); it is not known if this is due to DMPA, or to other related lifestyle factors which have a bearing on fracture rate. By contrast, in women using DMPA, the fracture risk before and after starting DMPA was not increased (relative risk 1.08, 95% CI 0.92-1.26). Importantly, this study could not determine whether use of DMPA has an effect on fracture rate later in life.
For further information on BMD changes in both adult and adolescent females, as reported in recent clinical studies, refer to section 5.1 (Pharmacodynamic Properties). Adequate intake of calcium and Vitamin D, whether from the diet or from supplements, is important for bone health in women of all ages.
Menstrual Irregularities:
Most women using ELASHINE experienced alteration of menstrual bleeding patterns. Patients should be appropriately counseled concerning the likelihood of menstrual disturbance and the potential delay in return to ovulation. As women continued using ELASHINE, fewer experienced irregular bleeding and more experienced amenorrhea. After receiving the fourth dose, 39% of women experienced amenorrhea during month 6. During month twelve, 56.5% of women experienced amenorrhea. The changes in menstrual patterns from the three contraception trials are presented in Figures 1 and 2. Figure 1 shows the increase in the percentage of women experiencing amenorrhea over the 12 month study. Figure 2 presents the percentage of women experiencing spotting only, bleeding only, and bleeding and spotting over the same time period. In addition to amenorrhea, altered bleeding patterns included intermenstrual bleeding, menorrhagia and metrorrhagia. If abnormal bleeding associated with ELASHINE persists or is severe, appropriate investigation and treatment should be instituted.
Figure 1. Percent of ELASHINE -Treated Women with Amenorrhea per 30-Day Month Contraception Studies (ITT Population, N=2053)
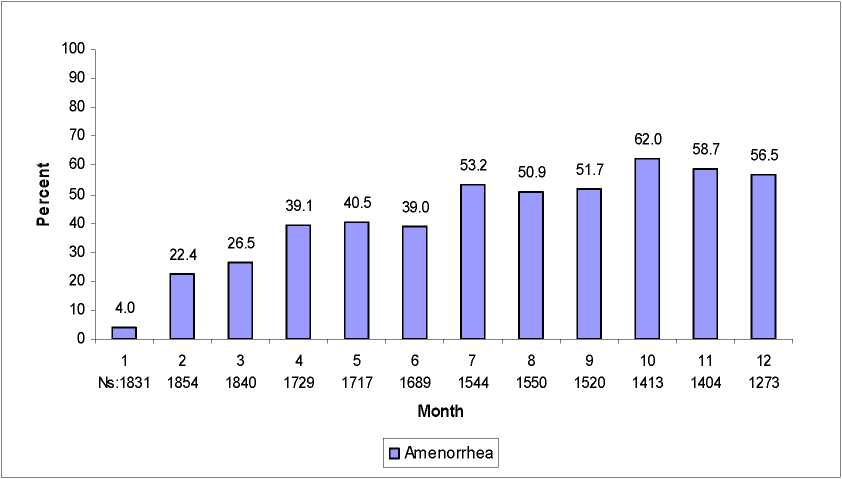
Figure 2. Percent of ELASHINE -Treated Women with Bleeding and/or Spotting per 30-Day Month Contraception Studies (ITT Population, N=2053)
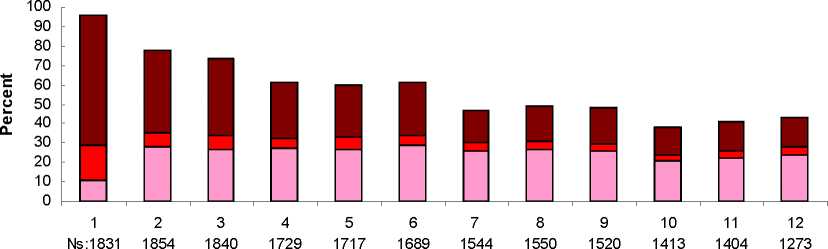
Month
□ Spotting Only □ Bleeding Only □ Bleeding and Spotting
Cancer Risks:
Long-term case-controlled surveillance of DMPA-IM 150 mg users found no overall increased risk of ovarian, liver, or cervical cancer and a prolonged, protective effect of reducing the risk of endometrial cancer in the population of users.
Breast cancer is rare among women under 40 years of age whether or not they use hormonal contraceptives.
Results from some epidemiological studies suggest a small difference in the risk of having the disease_in current and recent users compared with never-users. Any excess risk in current and recent DMPA users is small in relation to the overall risk of breast cancer, particularly in young women (see below), and is not apparent after 10 years since last use. Duration of use does not seem to be important.
Possible number of additional cases of breast cancer diagnosed up to 10 years after stopping injectable progestogens*
|
Age at last use of DMPA |
No of cases per 10,000 women who are never-users |
Possible additional cases per 10,000 DMPA users |
|
20 |
Less than 1 |
Much less than 1 |
|
30 |
44 |
2-3 |
|
40 |
160 |
10 |
*based on use for 5 years”
Thromboembolic Disorders
Although MPA has not been causally associated with the induction of thrombotic or thromboembolic disorders, any patient who develops such an event, e.g. pulmonary embolism, cerebrovascular disease, retinal thrombosis or deep venous thrombosis, while undergoing therapy with ELASHINE should not be readministered the drug. Women with a prior history of thromboembolic disorders have not been studied in clinical trials and no information is available that would support the safety of ELASHINE use in this population.
Anaphylaxis and Anaphylactoid Reaction
If an anaphylactic reaction occurs appropriate therapy should be instituted. Serious anaphylactic reactions require emergency medical treatment.
Ocular Disorders
Medication should not be re-administered pending examination if there is a sudden partial or complete loss of vision or if there is a sudden onset of proptosis, diplopia, or migraine. If examination reveals papilledema or retinal vascular lesions, medication should not be re-administered.
Precautions
Weight Changes
Weight changes are common but unpredictable. In the phase 3 studies body weight was followed over 12 months. Half (50%) of women remained within
2.2 Kg of their initial body weight. 12% of women lost more than 2.2 Kg, and 38% of women gained more than 2.3 Kg.
Fluid Retention
There is evidence that progestogens may cause some degree of fluid retention, and as a result, caution should be exercised in treating any patient with a preexisting medical condition that might be adversely affected by fluid retention.
Return of Ovulation
Following a single dose of ELASHINE, the cumulative rate of return to ovulation as measured by plasma progesterone was 97.4% (38/39 patients) by one year after administration. After the 14-week therapeutic window, the earliest return to ovulation was one week, and the median time to ovulation was 30 weeks. Women should be counseled that there is a potential for delay in return to ovulation following use of the method, regardless of the duration of use. It is recognised, however, that amenorrhoea and/or irregular menstruation upon discontinuation of hormonal contraception may be due to an underlying disorder associated with menstrual irregularity especially polycystic ovarian syndrome.
Psychiatric Disorders
Patients with a history of treatment for clinical depression should be carefully monitored while receiving ELASHINE.
Protection Against Sexually Transmitted Diseases
Patients should be counselled that ELASHINE does not protect against HIV infection (AIDS) or other sexually transmitted diseases.
Carbohydrate/Metabolism
Some patients receiving progestogens may exhibit a decrease in glucose tolerance. Diabetic patients should be carefully observed while receiving such therapy.
Liver Function
If jaundice develops in any woman receiving ELASHINE, consideration should be given to not re-administer the medication. (see section 4.3)
Hypertension and Lipid disorders
Limited evidence suggests that there is a small increased risk of cardiovascular events among women with hypertension or with lipid disorders who used progestogen-only injectables. If hypertension occurs under ELASHINE treatment and/or the increase in hypertension cannot adequately be controlled by antihypertensive medication, treatment with ELASHINE should be stopped. Additional risk factors for arterial thrombotic disorders include: Hypertension, smoking, age, lipid disorders, migraine, obesity, positive family history, cardiac valve disorders, atrial fibrillation.
ELASHINE should be used cautiously in patients with one or more of these risk factors.
Other conditions
The following conditions have been reported both during pregnancy and during sex steroid use, but an association with the use of progestagens has not been established: jaundice and/or pruritus related to cholestasis; gallstone formation; porphyria; systemic lupus erythematosus; hemolytic uraemic syndrome; Sydenham's chorea; herpes gestationis; otosclerosis-related hearing loss.
If any of the conditions/risk factors mentioned is present, the benefits of ELASHINE use should be weighed against the possible risks for each individual woman and discussed with the woman before she decides to start using it. In the event of aggravation, exacerbation or first appearance of any
of these conditions or risk factors, the woman should contact her physician. The physician should then decide on whether ELASHINE use should be discontinued.
Laboratory Tests
The pathologist should be advised of progestogen therapy when relevant specimens are submitted. The physician should be informed that certain endocrine and liver function tests, and blood components might be affected by progestogen therapy:
a) Plasma/urinary steroids are decreased (e.g. progesterone, estradiol, pregnanediol, testosterone, cortisol)
b) Plasma and urinary gonadotropin levels are decreased (e.g., LH, FSH).
c) Sex-hormone-binding-globulin (SHBG) concentrations are decreased.
Important information about excipients
As this product contains methylparahydroxybenzote (E218) and propylparahydroxybenzoate (E216), it may cause allergic reactions (possibly delayed), and exceptionally, bronchospasm. This medicinal product contains less than 1 mmol sodium (23 mg) per 104mg/0.65ml, i.e. essentially ‘sodium-free’ .
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed with ELASHINE.
Interactions with other medical treatments (including oral anticoagulants) have rarely been reported, but causality has not been determined. The possibility of interactions should be borne in mind in patients receiving concurrent treatment with other drugs.
MPA is metabolized in vitro primarily by hydroxylation via the CYP3A4. Specific drug-drug interaction studies evaluating the clinical effects with CYP3A4 inducers or inhibitors on MPA have not been conducted and therefore the clinical effects of CYP3A4 inducers or inhibitors are unknown.
4.6 Fertility, pregnancy and lactation
Fertility
ELASHINE is indicated for the prevention of pregnancy.
Women may experience a delay in return to fertility (conception) following discontinuation of ELASHINE (see section 4.4).
Pregnancy
ELASHINE is contraindicated in women who are pregnant. Some reports suggest an association between intrauterine exposure to progestational drugs in the first trimester of pregnancy and genital abnormalities in male and female fetuses. If ELASHINE is used during pregnancy, or if the patient becomes pregnant while using this drug, the patient should be warned of the potential hazard to the fetus.
One study found that infants from unintentional pregnancies that occurred 1 to 2 months after injection of medroxyprogesterone acetate Injection 150 mg IM were at an increased risk of low birth weight; this, in turn, has been associated with an increased risk of neonatal death. However, the overall risk of this is very low because pregnancies while on medroxyprogesterone acetate Injection 150 mg IM are uncommon.
Children exposed to MPA in utero and followed to adolescence showed no evidence of any adverse effects on their health including their physical, intellectual, sexual or social development.
Lactation
Low detectable amounts of drug have been identified in the milk of mothers receiving MPA. In nursing mothers treated with medroxyprogesterone acetate injection 150 mg IM, milk composition, quality, and amount are not adversely affected. Neonates and infants exposed to MPA from breast milk have been studied for developmental and behavioural effects through puberty. No adverse effects have been noted. However, due to limitations of the data regarding the effects of MPA in breastfed infants less than six weeks old, ELASHINE should be given no sooner than six weeks post partum when the infant’s enzyme system is more developed.
4.7 Effects on ability to drive and use machines
ELASHINE has no influence on the ability to drive and use machines.
4.8 Undesirable effects
Events from clinical trials:
The table below provides a listing of adverse drug reactions with frequency based on allcausality data from clinical studies that enrolled 2053 women who received DMPA-SC for contraception. The most frequently (>5%) reported adverse drug reactions were headache (8.9%), metrorrhagia (7.1%), weight increased (6.9%), amenorrhoea (6.3%) and injection site reactions (any type, 6.1%).
Adverse reactions are listed according to the following categories. These are as follows: Very Common (>1/10)
Common (>1/100 to <1/10)
Uncommon (>1/1,000 to <1/100)
Rare (>1/10,000 to <1/1,000)
Frequency not known (cannot be estimated from the available data)
Events from post-marketing surveillance:
In addition, adverse events of medical significance obtained from post-marketing data with
|
the use of injectable DMPA (IM |
or SC) are also included in the list below: | ||||
|
System organ class |
Very Common |
Common |
Uncommon |
Rare |
Not known |
|
Neoplasms benign, malignant and unspecified (including cysts and polyps) |
Breast cancer (see section 4.4) | ||||
|
Immune system disorders |
Drug hypersensitivity (see section 4.4) |
Anaphylactic reaction, Anaphylactoid reaction, Angioedema (see section 4.4) | |||
|
Metabolism and nutrition disorders |
Fluid retention (see section 4.4), Increased appetite, Decreased appetite | ||||
|
Psychiatric disorders |
Depression, Insomnia, Anxiety, Affective disorder, Irritability, Libido decreased |
Nervousness, Emotional disorder, Anorgasmia | |||
|
Nervous system disorders |
Dizziness, Headache |
Migraine, Somnolence |
Seizure | ||
|
Ear and labyrinth disorders |
Vertigo | ||||
|
Cardiac disorders |
Tachycardia | ||||
|
Vascular disorders |
Hypertension (see section 4.4), Varicose vein, Hot flush |
Pulmonary embolism, Embolism and thrombosis (see section 4.4), Thrombophlebitis | |||
|
Gastrointestinal disorders |
Abdominal pain, Nausea |
Abdominal distension | |||
|
Hepatobiliary disorders |
Jaundice, Hepatic function abnormal (see section 4.4) | ||||
|
Skin and subcutaneous tissue disorders |
Acne |
Alopecia, Hirsutism, Dermatitis, Ecchymosis, |
Lipo dystrophy acquired |
Skin striae | |
|
System organ class |
Very Common |
Common |
Uncommon |
Rare |
Not known |
|
Chloasma, Rash, Pruritus, Urticaria | |||||
|
Musculoskeletal and connective tissue disorders |
Back pain, Pain in extremity |
Arthralgia, Muscle spasms |
Osteoporosis, Osteoporotic fractures | ||
|
Reproductive system & breast disorders |
Menometrorrhagia, Metrorrhagia, Menorrhagia (see section 4.4), Dysmenorrhoea, Amenorrhoea, Vaginitis, Breast pain |
Ovarian cyst, Uterine haemorrhage (irregular, increase, decrease), Vaginal discharge, Dyspareunia, Galactorrhoea, Pelvic pain, Vulvovaginal dryness, Premenstrual syndrome, Breast tenderness, Breast enlargement | |||
|
General disorders and administration site conditions |
Fatigue, Injection site reaction, Injection site persistent atrophy/ Indentation/ dimpling, Injection site nodule/lump, Injection site pain/tenderness |
Pyrexia |
Asthenia | ||
|
Investigations |
Weight increased (see section 4.4), Smear cervix abnormal |
Bone density decreased (see section 4.4), Glucose tolerance decreased (see section 4.4), Hepatic enzyme abnormal |
Weight decrease d_(see section 4.4) |
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via Yellow Card Scheme Website: www.mhra.gov.uk/yellowcard.
4.9 Overdose
No positive action is required other than cessation of therapy.
PHARMACOLOGICAL PROPERTIES
5
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: progestogens, ATC Code: G03AC06
MPA is an analogue of 17 a-hydroxyprogesterone with anti-estrogenic, anti-androgenic and antigonadotrophic effects.
ELASHINE MPA injectable suspension inhibits the secretion of gonadotropins which, in turn, prevents follicular maturation and ovulation and causes thickening of cervical mucus which inhibits sperm entry into the uterus. These actions produce its contraceptive effect.
BMD Changes in Adult Women
A study comparing changes in BMD in women using ELASHINE with women using medroxyprogesterone acetate injection (150 mg IM) showed no significant differences in BMD loss between the two groups after two years of treatment. Mean percent changes in BMD in the ELASHINE group are listed in Table 1.
Table 1. Mean Percent Change from Baseline in BMD in Women Using ELASHINE by Skeletal Site
|
Time on Treatment |
Lumbar Spine |
Total |
Hip |
Femoral Neck | ||
|
N |
Mean % Change (95% CI) |
N |
Mean % Change (95% CI) |
N |
Mean % Change (95% CI) | |
|
1 year |
166 |
-2.7 (-3.1 to -2.3) |
166 |
-1.7 (-2.1 to -1.3) |
166 |
-1.9 (-2.5 to -1.4) |
|
2 year |
106 |
- 4.1 (-4.6 to -3.5) |
106 |
-3.5 (-4.2 to -2.7) |
106 |
-3.5 (-4.3 to -2.6) |
In another controlled, clinical study adult women using medroxyprogesterone acetate injection (150 mg IM) for up to 5 years showed spine and hip mean BMD decreases of 5-6%, compared to no significant change in BMD in the control group. The decline in BMD was more pronounced during the first two years of use, with smaller declines in subsequent years. Mean changes in lumbar spine BMD of -2.86%, -4.11%, -4.89%, -4.93% and -5.38% after 1, 2, 3, 4 and 5 years, respectively, were observed. Mean decreases in BMD of the total hip and femoral neck were similar. Please refer to Table 2 below for further details.
After stopping use of medroxyprogesterone acetate injection (150 mg IM), BMD increased towards baseline values during the post-therapy period. A longer duration of treatment was associated with a slower rate of BMD recovery.
Table 2. Mean Percent Change from Baseline in BMD in Adults by Skeletal Site and Cohort after 5 Years of Therapy with Medroxyprogesterone acetate 150 mg IM and after 2 Years Post-Therapy or 7 Years of Observation (Control)
|
Time in Study |
Spine |
Total Hip |
Femoral Neck | |||
|
Medroxyprog esterone acetate |
Control |
Medroxyprog esterone acetate |
Control |
Medroxyprog esterone acetate |
Control | |
|
Time in Study |
Spine |
Total Hip |
Femoral Neck | |||
|
Medroxyprog esterone acetate |
Control |
Medroxyprog esterone acetate |
Control |
Medroxyprog esterone acetate |
Control | |
|
5 years* |
n=33 -5.38% |
n=105 0.43% |
n=21 -5.16% |
n=65 0.19% |
n=34 -6.12% |
n=106 -0.27% |
|
7 years** |
n=12 -3.13% |
n=60 0.53% |
n=7 -1.34% |
n=39 0.94% |
n=13 -5.38% |
n=63 -0.11% |
*The treatment group consisted of women who received medroxyprogesterone acetate injection (150 mg IM) for 5 years and the control group consisted of women who did not use hormonal contraception for this time period.
** The treatment group consisted of women who received medroxyprogesterone acetate Injection (150 mg IM) for 5 years and were then followed up for 2 years post-use and the control group consisted of women who did not use hormonal contraceptive for 7 years.
BMD Changes in Adolescent Females (12-18 years)
Results from an open-label, non-randomised, clinical study of medroxyprogesterone acetate Injection (150 mg IM every 12 weeks for up to 240 weeks (4.6 years), followed by posttreatment measurements) in adolescent females (12-18 years) also showed that medroxyprogesterone acetate IM use was associated with a significant decline in BMD from baseline. Among subjects who received > 4 injections/60-week period, the mean decrease in lumbar spine BMD was - 2.1 % after 240 weeks (4.6 years); mean decreases for the total hip and femoral neck were -6.4 % and -5.4 %, respectively. Post-treatment follow-up showed that, based on mean values, lumbar spine BMD recovered to baseline levels approximately 1 year after treatment was discontinued and that hip BMD recovered to baseline levels approximately 3 years after treatment was discontinued. However, it is important to note that a large number of subjects discontinued from the study, therefore these results are based on a small number of subjects (n=71 at 60 weeks and n=25 at 240 weeks after treatment discontinuation). In contrast, a non-comparable cohort of unmatched, untreated subjects, with different baseline bone parameters from the DMPA users, showed mean BMD increases at 240 weeks of 6.4%, 1.7% and 1.9% for lumbar spine, total hip and femoral neck, respectively.
5.2 Pharmacokinetic properties
The pharmacokinetic parameters of MPA following a single SC injection of ELASHINE are shown in Table 1.
Table 1. Pharmacokinetic Parameters of MPA
After a Single SC Injection of ELASHINE in Healthy Women (n = 42)
|
C v-/max |
Tmax |
C91 (min) |
AUC0-91 |
AUCc.~ |
t/ | |
|
(ng/ml) |
(day) |
(ng/ml) |
(ngday/ml) |
(ngday/ml) |
(day) | |
|
Mean |
1.56 |
8.8 |
0.402 |
66.98 |
92.84 |
43 |
|
Min |
0.53 |
2.0 |
0.133 |
20.63 |
31.36 |
16 |
|
Max |
3.08 |
80.0 |
0.733 |
139.79 |
162.29 |
114 |
Cmax = peak serum concentration; Tmax = time when Cmax is observed; AUC0-91 = area under the concentrationtime curve over 91 days; E/2 = terminal half-life; 1 nanogram = 103 picogram.
General Characteristics
Absorption
MPA absorption from the SC injection site to achieve therapeutic levels is relatively prompt. The mean Tmax attained approximately one week after injection. The peak MPA concentrations (Cmax) generally range from 0.5 to 3.0 ng/ml with a mean Cmax of 1.5 ng/ml after a single SC injection.
Effect of Injection Site
ELASHINE was administered into the anterior thigh or the abdomen to evaluate effects on MPA concentration-time profile. MPA trough concentrations (Cmin; Day 91) were similar for the two injection locations, suggesting that injection site does not negatively affect the contraceptive efficacy.
Distribution
Plasma protein binding of MPA averages 86%. MPA binding occurs primarily to serum albumin; no binding of MPA occurs with SHBG.
Biotransformation
MPA is extensively metabolized in the liver by P450 enzymes. Its metabolism primarily involves ring A and/or side-chain reduction, loss of the acetyl group, hydroxylation in the 2-, 6-, and 21-positions or a combination of these positions, resulting in more than 10 metabolites.
Elimination
Residual MPA concentrations at the end of the dosing interval (3 months) of ELASHINE are generally below 0.5 ng/ml, consistent with its apparent terminal halflife of ~40 days after SC administration. Most MPA metabolites are excreted in the urine as glucuronide conjugates with only small amounts excreted as sulfates.
Linearity/non-linearity
Based on single-dose data, there was no evidence of non-linearity over the dose range of 50 to 150 mg after SC administration. The relationship between the AUC or the Cmin and the SC dose of MPA appeared to be linear. The mean Cmax did not change substantially with increasing dose
Race
There were no apparent differences in the pharmacokinetics and/or dynamics of MPA after SC administration of ELASHINE among women of all ethnic backgrounds studied. The pharmacokinetics/dynamics of MPA has been evaluated in Asian women in a separate study.
Effect of Body Weight
No dosage adjustment of ELASHINE is necessary based on body weight. The effect of body weight on the pharmacokinetics of MPA was assessed in a subset of women (n = 42, body mass index [BMI] ranged from 18.2 to 46.0 kg/m2). The AUC0-9J values for MPA were 68.5, 74.8, and 61.8 ng -day/ml in women with BMI categories of < 25 kg/m2, >25 to <30 kg/m2, and >30 kg/m2, respectively. The mean MPA Cmax was 1.65 ng/ml in women with BMI < 25 kg/m2, 1.76 ng/ml in women with BMI >25 to <30 kg/m2, and 1.40 ng/ml in women with BMI > 30 kg/m2, respectively. The range of MPA trough (Cmin) concentrations and the half-lives were comparable for the 3 BMI groups.
Pharmacokinetic/Pharmacodynamic Relationship(s)
From a pharmacodynamic perspective, the duration of ovulation suppression depends upon maintaining therapeutic MPA concentrations throughout the 13 week dosing interval.
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity and carcinogenic potential. Medroxyprogestrone acetate has been shown to have adverse effects on reproduction in animals and is contraindicated for use during pregnancy.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Macrogol 3350
Methyl parahydroxybenzoate (E 218)
Propyl parahydroxybenzoate (E 216)
Sodium Chloride Polysorbate 80
Monobasic Sodium Phosphate Monohydrate Disodium Phosphate Dodecahydrate Methionine Povidone
Hydrochloric Acid and/or Sodium Hydroxide for pH adjustment Water for Injection
6.2 Incompatibilities
Not applicable
6.3 Shelf life
Unopened: 5 years
Once opened: use immediately. Discard any unused portion.
6.4 Special precautions for storage
Do not refrigerate or freeze
6.5 Nature and contents of container
ELASHINE suspension for injection is supplied as a disposable pre-filled syringe (type 1 clear glass Ph.Eur.) containing 0.65ml with plunger stopper and tip cap (bromobutyl rubber).
The pack sizes are one pre-filled syringe with one 26G, 3/8" needle and six pre-filled syringes with six 26G, 3/8” needles.
Not all pack sizes may be marketed.
6.6 Special precautions for disposal
For single use only.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
Instructions for Use and Handling
Getting ready
Do not refrigerate. Ensure that the medication is at room temperature prior to injection (to ensure appropriate viscosity of the suspension). Make sure the following components (Diagrams 6 and 7) are available.
1
Prefilled syringe .....
plastic
needle
cover

needle
Diagram 6 Diagram 7
ELASHINE, as with other parenteral drug products, should be inspected visually for particulate matter and discoloration prior to administration.
Syringe preparation
Gently twist off the protective end cap from the needle to break the seal (Diagram 8). Set aside.
While holding the syringe firmly by the barrel pointing upward, shake it vigorously for at least 1 minute to thoroughly mix the medication (Diagram 9).
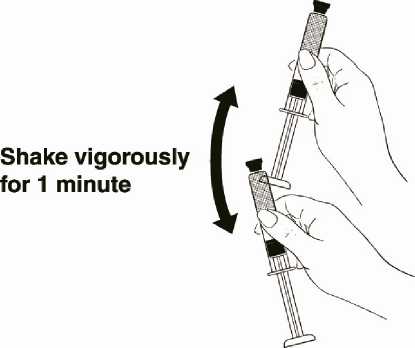
Shake vigorously for 1 minute
Then remove the protective cap from the tip of the syringe barrel. Diagram 9
While holding the syringe barrel, attach the needle to the barrel of the syringe firmly by pushing the plastic needle cover down fully with a slight twisting movement (Diagram 10).
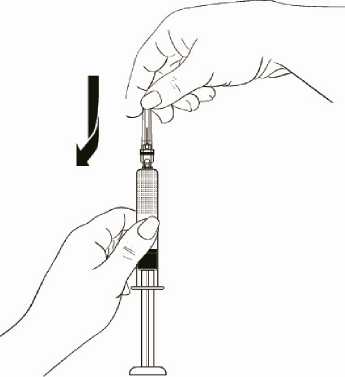
Diagram 10
7 MARKETING AUTHORISATION HOLDER
Pfizer Limited
Sandwich
Kent
CT13 9NJ United Kingdom
8 MARKETING AUTHORISATION NUMBER(S)
PL 00057/1500
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
23/12/2014
10 DATE OF REVISION OF THE TEXT
05/09/2016