Elumatic Iii Technetium Generator
SUMMARY OF PRODUCT CHARACTERISTICS 1 NAME OF THE MEDICINAL PRODUCT
ELUMATIC III 2 - 20 GBq radionuclide generator
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Sodium pertechnetate (99mTc) injection is produced by means of radioactive decay of molybdenum-99. Technetium-99m decays with the emission of gamma radiation with a mean energy of 140 keV and a half-life of 6.02 hours to technetium-99 which, in view of its long half-life of 2.13 x 105 years can be regarded as quasi stable.
The radionuclide generator containing the parent isotope 99Mo, adsorbed to a chromatographic column delivers sodium pertechnetate (99mTc) injection in sterile solution.
The 99Mo on the column is in equilibrium with the formed daughter isotope 99mTc. The generators are supplied with the following 99Mo activity amounts at activity reference time which deliver the following technetium (99mTc) amounts, assuming a 100% theoretical yield and 24 hours time from previous elution and taking into account that branching ratio of 99Mo is about 87%:
|
99mTc activity (Maximal theoretical eluable activity at calibration date, 12h CET) |
2 |
4 |
6 |
8 |
10 |
12 |
16 |
20 |
GBq |
|
99Mo activity (at calibration date, 12h CET) |
2.5 |
5 |
7 |
9.5 |
12 |
14.5 |
19 |
24 |
GBq |
Excipient with known effect:
Each mL of sodium pertechnetate (99mTc) solution contains 3.6 mg of sodium. For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Radionuclide generator
4 CLINICAL PARTICULARS
4.1
Therapeutic indications
This medicinal product is for diagnostic use only.
The eluate from the radionuclide generator (sodium pertechnetate (99mTc) injection), is indicated for:
• Labelling of various kits for radiopharmaceutical preparation developed and approved for radiolabelling with such solution.
• Thyroid scintigraphy: direct imaging and measurement of thyroid uptake to give information on the size, position, nodularity and function of the gland in case of thyroid disease.
• Salivary gland scintigraphy: diagnosis of chronic sialadenitis (e.g. Sjogren's Syndrom) as well as assessment of salivary gland function and duct patency in salivary glands disorders and monitoring of the response to therapeutic interventions (in particular radioiodine therapy).
• Location of ectopic gastric mucosa (Meckel's diverticulum).
• Lacrimal duct scintigraphy: to assess functional disorders of lacrimation and monitoring of the response to therapeutic interventions.
4.2 Posology and method of administration
Posology
If sodium pertechnetate (99mTc) is administered intravenously, activities may vary widely according to the clinical information required and the equipment employed. The injection of activities greater than local DRLs (Diagnostic Reference Levels) should be justified for certain indications. Recommended activities are as follows:
Adults (70 kg) and elderly _population
• Thyroid scintigraphy: 20 - 80 MBq
• Salivary gland scintigraphy: 30 to 150 MBq for static images up to 370 MBq for dynamic images
• Meckel's diverticulum scintigraphy: 300-400 MBq
• Lacrimal duct scintigraphy: 2 - 4 MBq per drop per eye Renal impairment
Careful consideration of the activity to be administered is required since an increased radiation exposure is possible in these patients.
Paediatric _ population
The use in children and adolescents has to be considered carefully, based upon clinical needs and assessing the risk/benefit ratio in this patient group.
The activity to be administered to children and adolescents must be adapted and may be calculated according to the recommendations of the European Association of Nuclear Medicine (EANM) paediatric dosage card, by multiplying a baseline activity (for calculation purposes) by the weight-dependent correction factor given in the table below (see Table 1).
A[MBq]Administered = Baseline Activity x Multiple Thyroid scintigraphy:
Activity administered [MBq] = 5.6 MBq x correction factor (Table 1).
A minimal activity of 10 MBq is necessary for obtaining images of sufficient quality.
Identification/location of ectopic gastric mucosa:
Activity administered [MBq] = 10.5 MBq x correction factor (Table 1)
A minimal activity of 20 MBq is necessary in order to obtain images of sufficient quality.
Table 1: Weight-dependent correction factors in the paediatric population (for
thyroid scintigraphy and identification/location of ectopic gastric mucosa) according to the EANM-May 2008 guidelines
|
Weight [kgl |
Multiple |
Weight [kgl |
Multiple |
Weight [kgl |
Multiple |
|
3 |
1 |
22 |
5.29 |
42 |
9.14 |
|
4 |
1.14 |
24 |
5.71 |
44 |
9.57 |
|
6 |
1.71 |
26 |
6.14 |
46 |
10.00 |
|
8 |
2.14 |
28 |
6.43 |
48 |
10.29 |
|
10 |
2.71 |
30 |
6.86 |
50 |
10.71 |
|
12 |
3.14 |
32 |
7.29 |
52-54 |
11.29 |
|
14 |
3.57 |
34 |
7.72 |
56-58 |
12.00 |
|
16 |
4.00 |
36 |
8.00 |
60-62 |
12.71 |
|
18 |
4.43 |
38 |
8.43 |
64-66 |
13.43 |
|
20 |
4.86 |
40 |
8.86 |
68 |
14.00 |
Salivary gland scintigraphy:
The Paediatric Task Group of EANM (1990) recommends that the activity to be administered to a child should be calculated from the minimal adult posology and adapted to the child body weight according to the table below (see Table 2) with a minimum activity of 10 MBq in order to obtain images of sufficient quality.
Table 2: Weight-dependent correction factor in the paediatric population (for
salivary gland scintigraphy) according to EANM 1990 recommendations
|
Weight [kgl |
factor |
Weight [kgl |
factor |
Weight [kgl |
factor |
|
3 |
0.10 |
22 |
0.50 |
42 |
0.78 |
|
4 |
0.14 |
24 |
0.53 |
44 |
0.80 |
|
6 |
0.19 |
26 |
0.56 |
46 |
0.82 |
|
8 |
0.23 |
28 |
0.58 |
48 |
0.85 |
|
10 |
0.27 |
30 |
0.62 |
50 |
0.88 |
|
12 |
0.32 |
32 |
0.65 |
52-54 |
0.90 |
|
14 |
0.36 |
34 |
0.68 |
56-58 |
0.92 |
|
16 |
0.40 |
36 |
0.71 |
60-62 |
0.96 |
|
18 |
0.44 |
38 |
0.73 |
64-66 |
0.98 |
|
20 |
0.46 |
40 |
0.76 |
68 |
0.99 |
Lacrimal duct scintigraphy:
Recommended activities apply as well for adults as for children.
Method of administration For intravenous or ocular use.
For multidose use.
For instructions on extemporaneous preparation of the medicinal product before administration, see section 12.
For patient preparation, see section 4.4.
In thyroid scintigraphy, salivary gland scintigraphy and identification/location of ectopic gastric mucosa, the sodium pertechnetate (99mTc) solution is administered by intravenous injection.
In lacrimal duct scintigraphy, drops are instilled in each eye (ocular use).
Image acquisition
Thyroid scintigraphy: 20 minutes after intravenous injection.
Salivary gland scintigraphy: immediately after intravenous injection and at regular intervals for 15 minutes.
Identification/location of ectopic gastric mucosa: immediately after intravenous injection and at regular intervals for 30 minutes.
Lacrimal duct scintigraphy: dynamic acquisition within 2 minutes after instillation, followed by static images acquired at regular intervals within 20 minutes.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
4.4 Special warnings and special precautions for use
Potential for hypersensitivity or anaphylactic reactions
If hypersensitivity or anaphylactic reactions occur, the administration of the medicinal product must be discontinued immediately and intravenous treatment initiated, if necessary. To enable immediate action in emergencies, the necessary medicinal products and equipment such as endotracheal tube and ventilator must be immediately available.
Individual benefit/risk justification
For each patient, the radiation exposure must be justifiable by the likely benefit. The activity administered should in every case be as low as reasonably achievable to obtain the required diagnostic information.
Renal impairment
Careful consideration of the benefit/risk ratio in these patients is required since an increased radiation exposure is possible.
Paediatric population
For information on the use in paediatric population, see section 4.2.
Careful consideration of the indication is required since the effective dose per MBq is higher than in adults (see section 11).
Thyroid blocking, except for thyroid scintigraphy, is of special importance in the paediatric patient population.
Patient preparation
Pre-treatment of patients with thyroid-blocking medicinal products may be necessary for certain indications.
The patient should be well hydrated before the start of the examination and urged to void as often as possible during the first hours after the examination in order to reduce radiation.
To avoid false positives or to minimise irradiation by reduction of pertechnetate accumulation in the thyroid and salivary glands, a thyroid blocking agent should be given prior to lacrimal duct scintigraphy or Meckel’s diverticulum scintigraphy. Conversely a thyroid blocking agent must NOT be used before thyroid, parathyroid or salivary glands scintigraphy.
Before the application of sodium pertechnetate (99mTc) solution for scintigraphy of Meckel’s diverticulum, the patient should keep an empty stomach for 3 to 4 hours to reduce intestinal peristalsis.
After in vivo labelling of erythrocytes using stannous ions for reduction sodium pertechnetate (99mTc) is primarily built into erythrocytes, therefore Meckel’s scintigraphy should be performed before or some days after in vivo labelling of erythrocytes.
After the procedure
Close contact with infants and pregnant women should be restricted during 12 hours. Specific warnings
Sodium pertechnetate (99mTc) solution for injection contains 3.6 mg/mL of sodium. Depending on the time when the injection is administered, the content of sodium given to the patient may in some cases be greater than 1 mmol (23 mg). This should be taken into account in patient on low sodium diet.
When sodium pertechnetate (99mTc) solution is used for labelling of a kit, the determination of the overall sodium content must take into account the sodium derived from the eluate and the kit. Please refer to the package leaflet of the kit.
In salivary gland scintigraphy a lower specificity of the method should be expected compared to magnetic resonance sialography.
For precautions with respect to environmental hazard, see section 6.6.
4.5 Interaction with other medicinal products and other forms of interaction
Atropine, isoprenaline and analgesics may cause a delay of gastric emptying and thereby cause a redistribution of (99mTc) pertechnetate in abdominal imaging.
Administration of laxatives should be withheld since they irritate the gastrointestinal tract. Contrast-enhanced studies (e.g. barium) and upper gastro-intestinal examination should be avoided within 48 h prior to administration of pertechnetate (99mTc) for Meckel’s diverticulum scintigraphy.
Many pharmacological medicinal products are known to modify the thyroid uptake.
• antithyroid medicinal products (e.g. carbimazole or other imidazole derivatives such as propylthiouracil), salicylates, steroids, sodium nitroprusside, sodium sulfobromophtalein, perchlorate should be withheld for 1 week prior thyroid scintigraphy;
• phenylbutazone and expectorants should be withheld for 2 weeks;
• natural or synthetic thyroid preparations (e.g. sodium thyroxine, sodium liothyronine, thyroid extract) should be withheld for 2-3 weeks;
• amiodarone, benzodiazepines, lithium should be withheld for 4 weeks; intravenous contrast agents should not have been administered within 1-2 months.
4.6 Fertility, pregnancy and lactation
Women of childbearing potential
When an administration of radiopharmaceuticals to a woman of childbearing potential is intended, it is important to determine whether or not she is pregnant. Any woman who has missed a period should be assumed to be pregnant until proven otherwise. If in doubt about her potential pregnancy (if the woman has missed a period, if the period is very irregular, etc.), alternative techniques not using ionising radiation (if there are any) should be offered to the patient.
Pregnancy
Administration of pertechnetate (99mTc) to a woman who is known to be pregnant should be justified by medical need and a positive individual benefit/risk assessment for the mother and the foetus. Alternative non-irradiating diagnostic modalities should be taken into account.
99mTc (as free pertechnetate) has been shown to cross the placental barrier. Breastfeeding
Before administering radiopharmaceuticals to a mother who is breastfeeding, consideration should be given to the possibility of delaying the administration of radionuclide until the mother has ceased breastfeeding, and to what is the most appropriate choice of radiopharmaceuticals, bearing in mind the secretion of activity in breast milk. If the administration is considered necessary, breastfeeding should be interrupted for 12 hours post administration and the expressed feeds discarded.
Close contact with infants should be restricted during this period.
4.7 Effects on ability to drive and use machines
Sodium pertechnetate (99mTc) solution has no influence on the ability to drive or use machines.
4.8 Undesirable effects
Summary of the safety profile
Information on adverse reactions is available from spontaneous reporting. The reported reaction types are anaphylactoid reactions, vegetative reactions, as well as different kinds of injection site reactions. Sodium pertechnetate (99mTc) from the Elumatic III radionuclide generator is used for radioactive labelling of a variety of compounds. These medicinal products generally have a higher potential for adverse reactions than 99mTc, and therefore the reported adverse reactions are rather related to the labelled compounds than to 99mTc. The possible types of adverse reactions following intravenous administration of a 9 mTc-labelled pharmaceutical preparation will be dependent on the specific compound being used. Such information can be found in the SmPC of the kit used for radiopharmaceutical preparation.
Tabulated list of adverse reactions
The frequencies of undesirable effects are defined as follows:
Not known (cannot be estimated from the available data).
Immune system disorders
Frequency not known*: Anaphylactoid reactions (e.g. dyspnoea, coma, urticaria, erythema, rash, pruritus, oedema at various location e.g. face oedema)
Nervous system disorders
Frequency not known*: Vasovagal reactions (e.g. syncope, tachycardia, bradycardia, dizziness, headache, vision blurred, flushing)
Gastrointestinal disorders
Frequency not known*: Vomiting, nausea, diarrhoea General disorders and administration site conditions
Frequency not known*: Injection site reactions due to extravasation (e.g. cellulitis, pain, erythema, swelling)
* Adverse reactions derived from spontaneous reporting
Exposure to ionising radiation is linked with cancer induction and a potential for development of hereditary defects. As the effective dose is 5.2 mSv when the maximal recommended activity of 400 MBq is administered these adverse reactions are expected to occur with a low probability.
Description of selected adverse reactions
Anaphylactic reactions (e.g. dyspnoea, coma, urticaria, erythema, rash, pruritus, oedema at various locations [e.g. face oedema]).
Anaphylactic reactions have been reported following intravenous injection of sodium pertechnetate (99mTc) and include various skin or respiratory symptoms like skin irritations, oedema or dyspnoea.
Vegetative reactions (nervous system and gastrointestinal disorders)
Single cases of severe vegetative reactions have been reported, however, most of the reported vegetative reactions include gastrointestinal reactions like nausea or vomiting. Other reports include vasovagal reactions like headache or dizziness. Vegetative reactions are rather considered to be related to the examinational setting than to technetium (99mTc), especially in anxious patients.
General disorders and administration site conditions
Other reports describe local injection site reactions. Such reactions are related to extravasation of the radioactive material during the injection, and the reported reactions rank from local swelling up to cellulitis. Depending on the administered radioactivity and the labelled compound, extended extravasation may necessitate surgical treatment.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme. Website: www.mhra.gov.uk/yellowcard.
4.9 Overdose
In the event of administration of a radiation overdose with sodium pertechnetate (99mTc), the absorbed dose should be reduced where possible by increasing the elimination of the radionuclide from the body by defecation, forced diuresis and frequent bladder voiding.
The uptake in the thyroid, salivary glands and the gastric mucosa can be significantly reduced when sodium or potassium perchlorate is given immediately after an accidentally high dose of sodium pertechnetate (99mTc) was administered.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Diagnostic radiopharmaceuticals, various thyroid diagnostic radiopharmaceuticals, ATC code: V09FX01.
No pharmacological activity has been observed in the range of doses administered for diagnostic purposes.
5.2 Pharmacokinetic properties
Distribution
The pertechnetate ion has similar biological distribution to iodide and perchlorate ions, concentrating temporarily in salivary glands, choroid plexus, stomach (gastric mucosa) and in the thyroid gland, from which it is eliminated, unchanged. The pertechnetate ion also tends to concentrate in areas with increased vascularisation or with abnormal vascular permeability, particularly when pre-treatment with blocking agents inhibits uptake in glandular structures. With intact blood brain barrier, sodium pertechnetate (99mTc) does not penetrate into the brain tissue.
Organ uptake
In the blood 70-80% of the intravenously injected sodium pertechnetate (99mTc) is bound to proteins, primarily in an unspecific way to albumin. The unbound fraction (20-30%) accumulates temporarily in thyroid and salivary glands, stomach and nasal mucous membranes as well as in the plexus chorioideus.
Sodium pertechnetate (99mTc) in contrast to iodine, nevertheless, is neither used for the thyroid hormone synthesis (organification), nor absorbed in the small intestine. In the thyroid the maximum accumulation, depending on functional status and iodine saturation (in euthyroidism approx. 0.3-3%, in hyperthyroidism and iodine depletion up to 25%) is reached about 20 min after injection and then decreases quickly. This also applies for the stomach mucous membrane parietal cells and the salivary glands acinar cells.
In contrast to the thyroid which releases sodium pertechnetate (99mTc) in the bloodstream the salivary glands and the stomach secrete sodium pertechnetate (99mTc) in the saliva and gastric juice, respectively. The accumulation by the salivary gland lies in the magnitude of 0.5% of the applied activity with the maximum reached after about 20 minutes. One hour after injection, the concentration in the saliva is about 1030 fold higher than in the plasma. The excretion can be accelerated by lemon juice or by stimulation of the parasympathetic nerve system, the absorption is reduced by perchlorate.
Elimination
Half-life in plasma is approximately 3 hours. Sodium pertechnetate (99mTc) is not metabolised in the organism. One fraction is eliminated very quickly renally, the rest more slowly via faeces, salivary and tear liquid. Excretion during the first 24 hours following administration is mainly urinary (approximately 25%) with faecal excretion occurring over the next 48 hours. Approximately 50% of the administered activity is excreted within the first 50 hours. When selective uptake of pertechnetate (99mTc) in glandular structures is inhibited by the pre-administration of blocking agents, excretion follows the same pathways but there is a higher renal clearance.
The above data are not valid when sodium pertechnetate (99mTc) is used for labelling of another radiopharmaceutical.
5.3 Preclinical safety data
There is no information on acute, subacute and chronic toxicity from single or repeated dose administration. The quantity of sodium pertechnetate (99mTc) administered during clinical diagnostic procedures is very small and, apart from allergic reactions, no other adverse reactions have been reported.
This medicinal product is not intended for regular or continuous administration.
Mutagenicity studies and long-term carcinogenicity studies have not been carried out.
Reproductive toxicity
Placental transfer of 99mTc from intravenously administered sodium pertechnetate (99mTc) has been studied in mice. The pregnant uterus was found to contain as much as 60% of the injected 99mTc when administered without perchlorate preadministration. Studies performed on pregnant mice during gestation, gestation and lactation, and lactation alone showed changes in progeny which included weight reduction, hairlessness and sterility.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
• Column system:
Aluminium oxide
• Bag of solution for elution:
Sodium chloride
Sodium nitrate Water for injection
• Elution vials:
Nitrogen under reduced pressure
6.2 Incompatibilities
This medicinal product must not be mixed with other medicinal products except those mentioned in section 12.
6.3 Shelf life
Generator: 20 days from manufacturing date.
The calibration date and the expiry date are stated on the label.
Sodium pertechnetate (99mTc) eluate: after elution, store in a refrigerator (2°C - 8°C) and use within 10 hours.
Elution vials: 2 years.
6.4 Special precautions for storage
Generator: Do not store above 25°C. Store preferably inside the specific lead shielding for storage and elution "PROTEC-ELU" (available on request), or behind a lead shielding of appropriate thickness.
Eluate: For storage conditions after elution of the medicinal product, see section 6.3.
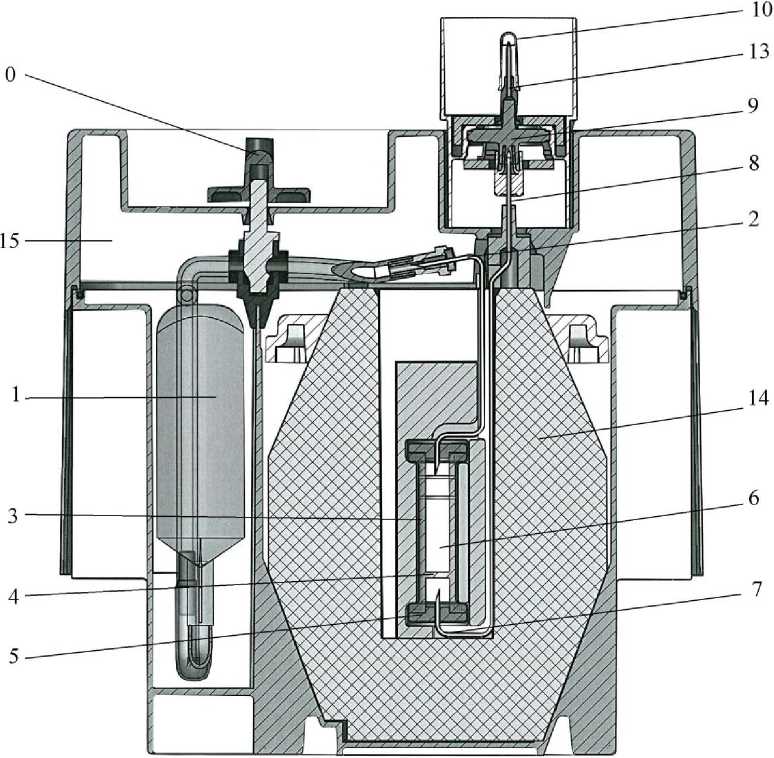
- A supple plastic bag (1) containing the eluent (0.9% sodium chloride and 0.005% sodium nitrate aqueous solution). The bag is connected by a stainless steel needle (2) to the top of the chromatographic column.
- A glass chromatographic column (3) with a filter at the bottom (4) to prevent any leakage of alumina. The column is obturated at both ends by caps maintained by metallic capsules (5). This column contains the alumina (6) which adsorbs the molybdate ions and is inert towards the pertechnetate ions.
- A needle with one end connected to the bottom of the column (7). The other end (8) is connected to a sterilising filtration assembly (9). The sterility of the elution needle (13) of the assembly is secured by a protective cap (10).
The column and the needles are protected by a cylindro-conical lead shielding (14) with a
minimal thickness of 52 mm. The whole system is placed in a parallelepipedic cover
(23 x 21 x 14 cm) made of moulded nylon (15).
Near the elution station is a cavity with a safety valve (0) turned off during the transport (O).
The generator is delivered in a tight metal drum and includes: - Ten sterilised needle caps, for single use only.
- A packet of ten single use 15 mL elution vials (TC-ELU 5), sterile, pyrogen-free and under partial vacuum allowing elution of 5 mL.
An elution container is supplied with the first order.
On request, it is possible to obtain kits containing vials of 15 mL:
- either under partial vacuum, allowing elution of 5 mL (Ref. TC-ELU-5);
- either under partial vacuum, allowing elution of 10 mL (Ref. TC-ELU-10);
- either under partial vacuum, allowing elution of 15 mL (Ref. TC-ELU-15).
Elution vials are 15 mL, colourless, European Pharmacopeia type I, drawn glass vial, closed with chlorobutyl rubber stoppers and aluminium capsules.
Packsize: One generator containing 2, 4, 6, 8, 10, 12, 16 or 20 GBq of sodium pertechnetate (99mTc) at calibration date.
6.6 Special precautions for disposal
General warnings
Radiopharmaceuticals should be received, used and administered only by authorised persons in designated clinical settings. Their receipt, storage, use, transfer and disposal are subject to the regulations and/or appropriate licences of the competent official organisation.
Radiopharmaceuticals should be prepared in a manner which satisfies both radiation safety and pharmaceutical quality requirements. Appropriate aseptic precautions should be taken.
If at any time the integrity of the generator or the vial with the eluted solution is compromised, it should not be used.
Administration procedures should be carried out in a way to minimise risk of contamination of the medicinal product and irradiation of the operators. Adequate shielding is mandatory.
The administration of radiopharmaceuticals creates risks for other persons from external radiation or contamination from spills of urine, vomiting, etc. Radiation protection precautions in accordance with national regulations must therefore be taken.
The residual activity of the generator must be estimated before disposal.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
CIS bio international B.P. 32
91192 Gif-sur-Yvette Cedex FRANCE
Tel. : +33-(0)1.69.85.70.70
Fax : +33-(0)1.69.85.70.71
8 MARKETING AUTHORISATION NUMBER(S)
PL 11876/0013
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
Date of first authorisation: 16 October 1997 Date of latest renewal: 21 September 2010
10 DATE OF REVISION OF THE TEXT
09/11/2016
11 DOSIMETRY
The data listed below are from ICRP 80 and are calculated according to the following assumptions:
i) Without pre-treatment with blocking agent:
|
Absorbed dose per administered unit of activity (mGy/MBq) | |||||
|
Organ |
Adult |
15 years |
10 years |
5 years |
1 year |
|
Adrenals |
0.0037 |
0.0047 |
0.0072 |
0.011 |
0.019 |
|
Bladder |
0.018 |
0.023 |
0.030 |
0.033 |
0.060 |
|
Bone surfaces |
0.0054 |
0.0066 |
0.0097 |
0.014 |
0.026 |
|
Brain |
0.0020 |
0.0025 |
0.0041 |
0.0066 |
0.012 |
|
Breast |
0.0018 |
0.0023 |
0.0034 |
0.0056 |
0.011 |
|
Gall bladder |
0.0074 |
0.0099 |
0.016 |
0.023 |
0.035 |
|
Gastro-intestinal tract | |||||
|
Stomach |
0.026 |
0.034 |
0.048 |
0.078 |
0.16 |
|
Small intestine |
0.016 |
0.020 |
0.031 |
0.047 |
0.082 |
|
Colon |
0.042 |
0.054 |
0.088 |
0.14 |
0.27 |
|
Upper large intestine |
0.057 |
0.073 |
0.12 |
0.20 |
0.38 |
|
Lower large intestine |
0.021 |
0.028 |
0.045 |
0.072 |
0.13 |
|
Heart |
0.0031 |
0.0040 |
0.0061 |
0.0092 |
0.017 |
|
Kidneys |
0.0050 |
0.0060 |
0.0087 |
0.013 |
0.021 |
|
Liver |
0.0038 |
0.0048 |
0.0081 |
0.013 |
0.022 |
|
Lungs |
0.0026 |
0.0034 |
0.0051 |
0.0079 |
0.014 |
|
Muscles |
0.0032 |
0.0040 |
0.0060 |
0.0090 |
0.016 |
|
Oesophagus |
0.0024 |
0.0032 |
0.0047 |
0.0075 |
0.014 |
|
Ovaries |
0.010 |
0.013 |
0.018 |
0.026 |
0.045 |
|
Pancreas |
0.0056 |
0.0073 |
0.011 |
0.016 |
0.027 |
|
Red bone marrow |
0.0036 |
0.0045 |
0.0066 |
0.0090 |
0.015 |
|
Salivary glands |
0.0093 |
0.012 |
0.017 |
0.024 |
0.039 |
|
Skin |
0.0018 |
0.0022 |
0.0035 |
0.0056 |
0.010 |
|
Spleen |
0.0043 |
0.0054 |
0.0081 |
0.012 |
0.021 |
|
Testes |
0.0028 |
0.0037 |
0.0058 |
0.0087 |
0.016 |
|
Thymus |
0.0024 |
0.0032 |
0.0047 |
0.0075 |
0.014 |
|
Thyroid |
0.022 |
0.036 |
0.055 |
0.12 |
0.22 |
|
Uterus |
0.0081 |
0.010 |
0.015 |
0.022 |
0.037 |
|
Remaining organs |
0.0035 |
0.0043 |
0.0064 |
0.0096 |
0.017 |
|
Effective Dose (mSv/MBq) |
0.013 |
0.017 |
0.026 |
0.042 |
0.079 |
(ii) With pre-treatment with blocking agent:
|
Absorbed dose per administered unit of activity (mGy/MBq) when blocking agents are administered | |||||
|
Organ |
Adult |
15 years |
10 years |
5 years |
1 year |
|
Adrenals |
0.0029 |
0.0037 |
0.0056 |
0.0086 |
0.016 |
|
Bladder |
0.030 |
0.038 |
0.048 |
0.050 |
0.091 |
|
Bone surfaces |
0.0044 |
0.0054 |
0.0081 |
0.012 |
0.022 |
|
Brain |
0.0020 |
0.0026 |
0.0042 |
0.0071 |
0.012 |
|
Breast |
0.0017 |
0.0022 |
0.0032 |
0.0052 |
0.010 |
|
Gall bladder |
0.0030 |
0.0042 |
0.0070 |
0.010 |
0.013 |
|
Gastro-intestinal tract | |||||
|
Stomach |
0.0027 |
0.0036 |
0.0059 |
0.0086 |
0.015 |
|
Small intestine |
0.0035 |
0.0044 |
0.0067 |
0.010 |
0.018 |
|
Colon |
0.0036 |
0.0048 |
0.0071 |
0.010 |
0.018 |
|
Upper large intestine |
0.0032 |
0.0043 |
0.0064 |
0.010 |
0.017 |
|
Lower large intestine |
0.0042 |
0.0054 |
0.0081 |
0.011 |
0.019 |
|
Heart |
0.0027 |
0.0034 |
0.0052 |
0.0081 |
0.014 |
|
Kidneys |
0.0044 |
0.0054 |
0.0077 |
0.011 |
0.019 |
|
Liver |
0.0026 |
0.0034 |
0.0053 |
0.0082 |
0.015 |
|
Lungs |
0.0023 |
0.0031 |
0.0046 |
0.0074 |
0.013 |
|
Muscles |
0.0025 |
0.0031 |
0.0047 |
0.0072 |
0.013 |
|
Oesophagus |
0.0024 |
0.0031 |
0.0046 |
0.0075 |
0.014 |
|
Ovaries |
0.0043 |
0.0054 |
0.0078 |
0.011 |
0.019 |
|
Pancreas |
0.0030 |
0.0039 |
0.0059 |
0.0093 |
0.016 |
|
Red bone marrow |
0.0025 |
0.0032 |
0.0049 |
0.0072 |
0.013 |
|
Skin |
0.0016 |
0.0020 |
0.0032 |
0.0052 |
0.0097 |
|
Spleen |
0.0026 |
0.0034 |
0.0054 |
0.0083 |
0.015 |
|
Testes |
0.0030 |
0.0040 |
0.0060 |
0.0087 |
0.016 |
|
Thymus |
0.0024 |
0.0031 |
0.0046 |
0.0075 |
0.014 |
|
Thyroid |
0.0024 |
0.0031 |
0.0050 |
0.0084 |
0.015 |
|
Uterus |
0.0060 |
0.0073 |
0.011 |
0.014 |
0.023 |
|
Remaining organs |
0.0025 |
0.0031 |
0.0048 |
0.0073 |
0.013 |
|
Effective Dose (mSv/MBq) |
0.0042 |
0.0054 |
0.0077 |
0.011 |
0.019 |
- (iii) The radiation doses absorbed by a patient following intravenous injection of (99mTc )-labelled red blood cells are as follows:
|
Absorbed dose per administered unit activity (mGy/MBq) | |||||
|
Organ |
Adult |
15 years |
10 years |
5 years |
1 year |
|
Adrenals |
0.0099 |
0.012 |
0.02 |
0.03 |
0.056 |
|
Bladder |
0.0085 |
0.011 |
0.014 |
0.017 |
0.031 |
|
Bone surfaces |
0.0074 |
0.012 |
0.019 |
0.036 |
0.074 |
|
Brain |
0.0036 |
0.0046 |
0.0075 |
0.012 |
0.022 |
|
Breast |
0.0035 |
0.0041 |
0.007 |
0.011 |
0.019 |
|
Gall bladder |
0.0065 |
0.0081 |
0.013 |
0.02 |
0.03 |
|
Gastrointestinal tract | |||||
|
Stomach |
0.0046 |
0.0059 |
0.0097 |
0.014 |
0.025 |
|
Small intestine |
0.0039 |
0.0049 |
0.0078 |
0.012 |
0.021 |
|
Colon |
0.0037 |
0.0048 |
0.0075 |
0.012 |
0.02 |
|
Upper large intestine |
0.004 |
0.0051 |
0.008 |
0.013 |
0.022 |
|
Lower large intestine |
0.0034 |
0.0044 |
0.0069 |
0.01 |
0.018 |
|
Heart |
0.023 |
0.029 |
0.043 |
0.066 |
0.11 |
|
Kidneys |
0.018 |
0.022 |
0.036 |
0.057 |
0.11 |
|
Liver |
0.013 |
0.017 |
0.026 |
0.04 |
0.072 |
|
Lungs |
0.018 |
0.022 |
0.035 |
0.056 |
0.11 |
|
Muscles |
0.0033 |
0.004 |
0.0061 |
0.0094 |
0.017 |
|
Oesophagus |
0.0061 |
0.007 |
0.0098 |
0.015 |
0.023 |
|
Ovaries |
0.0037 |
0.0048 |
0.007 |
0.011 |
0.019 |
|
Pancreas |
0.0066 |
0.0081 |
0.013 |
0.019 |
0.033 |
|
Red bone marrow |
0.0061 |
0.0076 |
0.012 |
0.02 |
0.037 |
|
Skin |
0.002 |
0.0024 |
0.0038 |
0.0062 |
0.012 |
|
Spleen |
0.014 |
0.017 |
0.027 |
0.043 |
0.081 |
|
Testes |
0.0023 |
0.003 |
0.0044 |
0.0069 |
0.013 |
|
Thymus |
0.0061 |
0.007 |
0.0098 |
0.015 |
0.023 |
|
Thyroid |
0.0057 |
0.0071 |
0.012 |
0.019 |
0.036 |
|
Uterus |
0.0039 |
0.0049 |
0.0074 |
0.011 |
0.019 |
|
Remaining organs |
0.0035 |
0.0045 |
0.0073 |
0.013 |
0.023 |
|
Effective Dose (mSv/MBq) |
0.007 |
0.0089 |
0.014 |
0.021 |
0.039 |
(iv) The radiation dose absorbed by the lens of the eye following administration of sodium pertechnetate (99mTc)for lachrymal duct scintigraphy is estimated to be 0.038 mGy/MBq. This results in an effective dose equivalent of less than 0.01 mSv for an administered activity of 4 MBq.
12 INSTRUCTIONS FOR PREPARATION OF RADIOPHARMACEUTICALS
Usual precautions regarding sterility and radiation safety should be respected.
Method of preparation
Disinfect the stopper of elution vials before each elution.
Warning
Do not use ethanol or ethyl ether to disinfect the needle or the stopper of the elution vial, as this may interfere with the elution process.
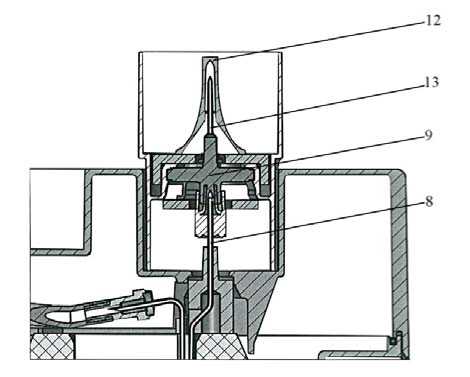
Between two elutions, protect the elution needle (13) from possible bacterial contamination by placing one of the ten sterilised needle caps (12) over this needle.
Observe the following sequences to obtain satisfactory results
First elution:
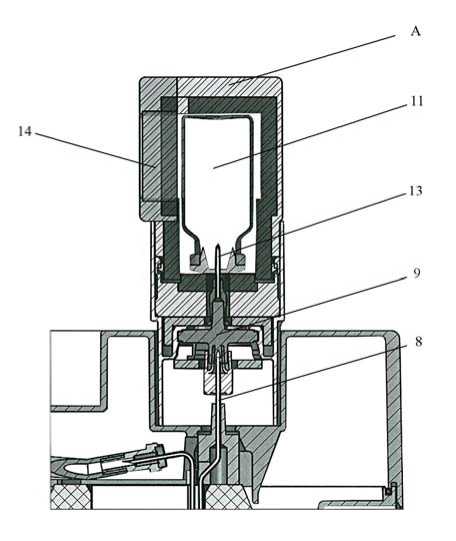
When starting using the generator, OPEN the safety valve (n° 0 : ), BEFORE
putting the elution vial in place. NEVER turn off the valve between two elutions.
Turn it off only when the generator is not being used any more.
To elute the generator, replace the protective cap of the elution needle (10) by the elution container (A) with an elution vial (11) corresponding to the elution volume required.
The elution may be observed through the lead glass window (14) of container (A). Wait forat least 3 minutes for total elution.
Before use, check the clarity of the eluate. If the eluate is not clear, it should be discarded.
After elution, immediately replace container (A) with the elution vial by one of the sterilised needle caps (12) over the needle.
Elution volumes
The generator ELUMATIC III® is designed to elute all the available technetium-99m activity in 5 ml. Fractionated elutions are therefore unnecessary. On the other hand, elution may be performed in larger volumes such as 10 or 15 ml.
Possibilities of use
The activity quoted on the label of the ELUMATIC III® is expressed in available technetium-99m at the calibration time (12 h CET).
The available activity of technetium-99m depends on:
- the molybdenum-99 activity at the time of elution;
- the time elapsed since the last elution was performed.
Activities of technetium-99m available with elutions performed every 24 hours can be calculated with table 4:
|
Previous days |
Calibration date | |||||||
|
-8 |
-7 |
-6 |
-5 |
-4 |
-3 |
-2 |
-1 |
0 |
|
751 |
584 |
454 |
353 |
274 |
213 |
166 |
129 |
100 |
|
Available activity in percent of 99mTc at the calibration date (round values) | ||||||||
|
Calibration date |
Following days | |||||||||||||
|
0 |
+1 |
+2 |
+3 |
+4 |
+5 |
+6 |
+7 |
+8 |
+9 |
+10 |
+11 |
+12 |
+13 |
+14 |
|
100 |
78 |
60 |
47 |
36 |
28 |
22 |
17 |
13 |
10 |
8 |
6 |
5 |
4 |
3 |
|
Available activity in percent of 99mTc at the calibration date (round values) | ||||||||||||||
TABLE 4
It is also possible, to elute the ELUMATIC III® before 24 hours have elapsed thus performing "partial time" elutions. Table 5 shows the percentage of activity in technetium-99m which can be collected after times varying from 0 to 23 hours :
|
Time elapsed since the last elution was performed (hours) |
0 |
1 |
2 |
3 |
4 |
5 |
6 |
8 |
10 |
12 |
14 |
16 |
18 |
20 |
22 |
23 |
|
Corrective factor |
0.00 |
0.11 |
0.21 |
0.30 |
0.39 |
0.45 |
0.51 |
0.62 |
0.71 |
0.79 |
0.85 |
0.89 |
0.93 |
0.96 |
0.99 |
1.00 |
|
Decay of 99Mo (see decay table inside cover) |
100 |
98.95 |
97.92 |
96.90 |
95.89 |
94.88 |
93.97 |
91.94 |
90.03 |
88.16 |
86.33 |
84.53 |
82.78 |
81.05 |
79.37 |
78.54 |
|
% of 99mTc available (round values) |
0 |
11 |
21 |
29 |
37 |
43 |
48 |
57 |
64 |
70 |
73 |
75 |
77 |
78 |
79 |
79 |
|
Available activity in percent of 99mTc activity at the time of previous elution (if performed about 24 hours after the previous one) | ||||||||||||||||
TABLE 5
Examples
a) A 10 GBq generator is eluted 24 hours after the calibration date. The technetium-99m activity collected is (table 4):
10 x 78= 7.8 GBq 100
b) The same generator is eluted 6 hours later. The technetium-99m activity collected is (tables 4 and 5):
7.8 x 48= 3.7 GBq 100
c) The same generator is eluted 18 hours later i.e. 48 hours after the calibration date. The 24 hours needed to reach the 99Mo-99mTc equilibrium have not elapsed and the technetium-99m activity collected will be instead of 6.0 GBq (tables 4 and 5: corrective factor):
6.0 x 93= 5.6 GBq 100
This is summarised in the following table:
|
Monday |
Tuesday |
Wednesday |
Thursday |
Friday | |||
|
Time of elution |
8 a.m. |
8 a.m. |
8 a.m. |
8 a.m. |
8 a.m. | ||
|
Radioactivity eluted 10 GBq on Tuesday 270 mCi on Tuesday |
13 350 |
10 270 |
7.8 211 |
6.0 162 |
4.7 127 | ||
|
Time of elution |
8 a.m. |
8 a.m. |
8 a.m. |
2 p.m. |
8 a.m. |
12 a.m. |
8 a.m. |
|
Same generator eluted at different times (GBq) (mCi) |
13 350 |
10 270 |
7.8 211 |
3.7 101 |
5.6 151 |
2.1 56 |
4.5 122 |
TABLE 6
N. B.:
In case the user waits for 48 hours or more between two elutions, he will obtain the activity indicated in table 4 multiplied by 1.1 (this factor accounts for the "rate equilibrium" which appears after 48 hours between molybdenum-99 and technetium-99m). This remark applies mainly:
- to the first elution: the previous elution was performed in the production laboratory, several days before;
- when the generator has a high activity.
Interest of partial time elutions
The potential utilisation of a generator can be notably increased by partial time elutions. The ELUMATIC III® has the advantage of a small elution volume. When choosing an appropriate volume for the elution vial, the desired volumic activity can be obtained even when the period of time between two elutions is of a few hours.
Example:
An elution of 10 GBq has been performed at 10 a.m. in 15 ml. The volumic activity is 0.67 GBq/ml. A new elution performed at 2 p.m., 4 hours after the first one, will give 3.7 GBq. If this activity is collected in 5 ml instead of 15 ml as previously, the volumic activity,
O. 74 GBq/ml will be higher than in the morning.
Table 7 shows that a comparatively constant volumic activity can be obtained all along the week:
|
Calibration date |
Elutions on following days | |||||
|
0 |
+ 1 |
+ 2 |
+ 3 |
+ 4 |
+ 5 | |
|
Eluted activity GBq |
10 |
7.8 |
6.0 |
4.7 |
3.6 |
2.8 |
|
Elution volume ml |
15 |
15 |
10 |
8* |
5 |
5 |
|
Volumic activity GBq/ml |
0.67 |
0.52 |
0.60 |
0.59 |
0.72 |
0.56 |
TABLE 7
* To reach a final volume of 8 ml, 3 ml of 0.9 % sodium chloride injection are added to the 5 ml eluted in a vial TC-ELU-5.
Quality control
The user laboratory should control: clarity of the solution, pH, radioactivity, gamma spectrum.
To obtain an approximate estimate of molybdenum-99, prior to use of the injection, take a volume of eluate equivalent to 37 MBq and determine the gamma-ray spectrum using a sodium iodide detector with a shield of lead, of thickness 6 mm, interposed between the sample and the detector. The response in the region corresponding to the 0,740 MeV photon of molybdenum-99 does not exceed that obtained using 37 kBq of a standardised solution of molybdenum-99 measured under the same conditions, when all measurements are calculated with reference to the date and hour of administration.
Warning:
The maximal radioactivity contained in the generator at the time of reception can be higher than that indicated on the label on the corresponding calibration date. Refer to table 3, showing the maximal radioactivity of elutable sodium pertechnetate (99mTc) for each content of generator, to determine the maximal radioactivity contained in the generator at the time of reception.
Weight of (99mTc + 99Tc) in the eluate
The molybdenum-99 is transformed into technetium-99m (87.6 % of the molybdenum-99 disintegrations) and technetium-99 (12.4 % of the molybdenum-99 disintegrations). Thus, the eluted solution is not "carrier free". The calculation of the total weight (99Tc + 99mTc) expressed in pg present in the eluate can be done with the following simplified formula:
W (pg) =99mTc activity in the eluate x k F
k
5.161.10-3 when activity is expressed in GBq.
F is the ratio between the number of technetium-99m (N99m) and the total number of technetium atoms (Nt) :
F = N99m Nt
The values of this ratio in terms of time elapsed between two elutions are given in the table hereunder:
|
Hours |
Days | ||||||
|
0 |
1 |
2 |
3 |
4 |
5 |
6 | |
|
0 |
- |
0.277 |
0.131 |
0.076 |
0.0498 |
0.0344 |
0.0246 |
|
3 |
0.727 |
0.248 |
0.121 |
0.072 |
0.0474 |
0.0329 |
0.0236 |
|
6 |
0.619 |
0.223 |
0.113 |
0.068 |
0.0452 |
0.0315 |
0.0227 |
|
9 |
0.531 |
0.202 |
0.105 |
0.064 |
0.0431 |
0.0302 |
0.0218 |
|
12 |
0.459 |
0.184 |
0.098 |
0.061 |
0.0411 |
0.0290 |
0.0210 |
|
15 |
0.400 |
0.168 |
0.092 |
0.058 |
0.0393 |
0.0278 |
0.0202 |
|
18 |
0.352 |
0.154 |
0.086 |
0.055 |
0.0375 |
0.0266 |
0.0194 |
|
21 |
0.311 |
0.141 |
0.081 |
0.052 |
0.0359 |
0.0256 |
0.0187 |
TABLE 8
Examples:
1) The technetium-99m from an ELUMATIC III has been eluted in 5 ml ; the activity measured is 10 GBq; the previous elution was performed 27 hours earlier. The weight of technetium carrier will be:
W fog) = 10 x 5.161.10-3 = 0.208 ^g
0.248
corresponding to 0.042 ^g/ml.
2) The technetium-99m is eluted from an ELUMATIC III 4 days after the preparation, this being the first elution for the user. For an activity of 10 GBq eluted in 5ml, the weight of technetium carrier is:
W fog) = 10 x 5.161.10-3 = 1.036 ^g
0.0498
corresponding to 0.207 ^g/ml, which is 5 times as much carrier as in the former example. However small, this amount of technetium may affect the labelling yield of some compounds.
This remark applies not only to the ELUMATIC III but to all technetium-99m generators.
Table 9 shows the variation in the weight of technetium carrier on a 10 GBq generator from Tuesday and eluted every day at an interval of 24 hours, assuming that the first elution was performed 3 days after that performed on Monday.
|
Monday |
Tuesday |
Wednesday |
Thursday |
Friday | |
|
Radioactivity eluted GBq |
13 |
10 |
7.8 |
6.0 |
4.7 |
|
Weight of technetium carrier in qg for the whole eluate |
0.883 |
0.186 |
0.145 |
0.112 |
0.088 |
TABLE 9
Physical characteristics
Technetium-99m is produced by means of radioactive decay of molybdenum-99. Technetium-99m decays with the emission of gamma radiation with a mean energy of 140 keV and a halflife of 6.02 hours to technetium-99 which, in view of its long half-life of 2.13 x 105 years can be regarded as quasi stable.
Decay table for 99Mo (half-life : 66 hours)
DaysHours
%
DaysHours%
-8 d -192 750.82 -190 735.22 -188 719.94 -186 704.96 -184 690.33 -182 675.98 -180 661.94 -178 648.18 -176 634.71 -174 621.52 -172 608.61 -170 595.96
-7 d-168 -166 -164 -162 -160 -158 -156 -154 -152 -150 -148 -146
-6 d-144 -142 -140 -138 -136 -134 -132 -130 -128 -126 -124 -122
-5 d-120 -118 -116 -114 -112 -110 -108 -106 -104 -102 -100 -98
583.57 571.45
559.57 547.94
536.56 525.41 514.49
503.80 493.33 483.07
473.04
463.21
453.58 444.15 434.92 425.89
417.04 408.37 399.88
391.57 383.44 375.47 367.66
360.02
352.54
345.22
338.04
331.02 324.14 317.40
310.81 304.35
298.02
291.83 285.77
279.83
-4 d -96 -94 -92 -90 -88 -86 -84 -82 -80 -78 -76 -74
-3 d -72
-70
-68
-66
-64
-62
-60
-58
-56
-54
-52
-50
-2 d -48 -46 -44 -42 -40 -38 -36 -34 -32 -30 -28 -26
-1 d -24 -22 -20 -18 -16 -14 -12 -10 -8 -6 -4 -2
274.01
268.32
262.74
257.28
251.93 246.70
241.57
236.55
231.64 226.82 222.11
217.49
213.01
208.58 204.25 200.00
195.84 191.77 187.79 183.88 180.06
176.32
172.65
169.06
165.55 162.11
158.74 155.44 152.21 149.05 145.95 142.91
139.94 137.04 134.19 131.40
128.67
125.99
123.37
120.81
118.30
115.84 113.43
111.07 108.76
106.50
104.29 102.12
1
3
|
Hours % |
DaysHours% |
DaysHours% |
DaysHours% | |||||
|
- 0 |
100.00 |
4 |
d96 |
36.49 |
8 d192 |
13.31 |
12 d288 |
4.86 |
|
te2 |
97.92 |
98 |
35.73 |
194 |
13.04 |
290 |
4.76 | |
|
4 |
95.89 |
100 |
34.99 |
196 |
12.77 |
292 |
4.66 | |
|
6 |
93.89 |
102 |
34.26 |
198 |
12.50 |
294 |
4.56 | |
|
8 |
91.94 |
104 |
33.55 |
200 |
12.24 |
296 |
4.47 | |
|
10 |
90.03 |
106 |
32.85 |
202 |
11.99 |
298 |
4.38 | |
|
12 |
88.16 |
108 |
32.17 |
204 |
11.74 |
300 |
4.29 | |
|
14 |
86.33 |
110 |
31.50 |
206 |
11.49 |
302 |
4.20 | |
|
16 |
84.53 |
112 |
30.84 |
208 |
11.25 |
304 |
4.11 | |
|
18 |
82.78 |
114 |
30.20 |
210 |
11.02 |
306 |
4.02 | |
|
20 |
81.06 |
116 |
29.57 |
212 |
10.79 |
308 |
3.94 | |
|
22 |
79.37 |
118 |
28.96 |
214 |
10.57 |
310 |
3.86 | |
|
d24 |
77.72 |
5 |
d120 |
28.36 |
9 d216 |
10.35 |
13 d312 |
3.78 |
|
26 |
76.10 |
122 |
27.77 |
218 |
10.13 |
314 |
3.70 | |
|
28 |
74.52 |
124 |
27.19 |
220 |
9.92 |
316 |
3.62 | |
|
30 |
72.97 |
126 |
26.63 |
222 |
9.72 |
318 |
3.55 | |
|
32 |
71.46 |
128 |
26.07 |
224 |
9.51 |
320 |
3.47 | |
|
34 |
69.97 |
130 |
25.53 |
226 |
9.32 |
322 |
3.40 | |
|
36 |
98.52 |
132 |
25.00 |
228 |
9.12 |
324 |
3.33 | |
|
38 |
67.09 |
134 |
24.48 |
230 |
8.93 |
326 |
3.26 | |
|
40 |
65.70 |
136 |
23.97 |
232 |
8.75 |
328 |
3.19 | |
|
42 |
64.33 |
138 |
23.47 |
234 |
8.56 |
330 |
3.13 | |
|
44 |
63.00 |
140 |
22.99 |
236 |
8.39 |
332 |
3.06 | |
|
46 |
61.69 |
142 |
22.51 |
238 |
8.21 |
334 |
3.00 | |
|
d48 |
60.40 |
6 |
d144 |
22.04 |
10 d240 |
8.04 |
14 d 336 |
2.94 |
|
50 |
59.15 |
146 |
21.58 |
242 |
7.87 | |||
|
52 |
57.92 |
148 |
21.13 |
244 |
7.71 | |||
|
54 |
56.872 |
150 |
20.69 |
246 |
7.55 | |||
|
56 |
55.54 |
152 |
20.26 |
248 |
7.39 | |||
|
58 |
54.38 |
154 |
19.84 |
250 |
7.24 | |||
|
60 |
53.25 |
156 |
19.43 |
252 |
7.09 | |||
|
62 |
52.15 |
158 |
19.03 |
254 |
6.94 | |||
|
64 |
51.06 |
160 |
18.63 |
256 |
6.80 | |||
|
66 |
50.00 |
162 |
18.24 |
258 |
6.66 | |||
|
68 |
48.96 |
164 |
17.86 |
260 |
6.52 | |||
|
70 |
47.94 |
166 |
17.49 |
262 |
6.38 | |||
|
d72 |
46.95 |
7 |
d168 |
17.13 |
11 d264 |
6.25 | ||
|
74 |
45.97 |
170 |
16.77 |
266 |
6.12 | |||
|
76 |
45.02 |
172 |
16.42 |
268 |
5.99 | |||
|
78 |
44.08 |
174 |
16.08 |
270 |
5.87 | |||
|
80 |
43.16 |
176 |
15.75 |
272 |
5.75 | |||
|
82 |
42.27 |
178 |
15.42 |
274 |
5.63 | |||
|
84 |
41.39 |
180 |
15.10 |
276 |
5.51 | |||
|
86 |
40.53 |
182 |
14.79 |
278 |
5.40 | |||
|
88 |
39.69 |
184 |
14.48 |
280 |
5.28 | |||
|
90 |
38.86 |
186 |
14.18 |
282 |
5.17 | |||
|
92 |
38.05 |
188 |
13.88 |
284 |
5.07 | |||
|
94 |
37.26 |
190 |
13.60 |
286 |
4.96 | |||
TABLE 10
Decay table for 99mTc (half-life : 6.02 hours) :
|
H.Min |
% |
H.Min |
% |
H.Min |
% |
H.Min |
% |
H.Min |
% |
H.Min |
% |
|
0.05 |
99.05 |
2.05 |
78.67 |
4.05 |
62.49 |
6.05 |
49.64 |
8.05 |
39.43 |
10.05 |
31.32 |
|
0.10 |
98.10 |
2.10 |
77.92 |
4.10 |
61.89 |
6.10 |
49.16 |
8.10 |
39.05 |
10.10 |
31.02 |
|
0.15 |
97.16 |
2.15 |
77.18 |
4.15 |
61.30 |
6.15 |
48.69 |
8.15 |
38.68 |
10.15 |
30.72 |
|
0.20 |
96.23 |
2.20 |
76.44 |
4.20 |
60.72 |
6.20 |
48.23 |
8.20 |
38.31 |
10.20 |
30.43 |
|
0.25 |
95.32 |
2.25 |
75.71 |
4.25 |
60.14 |
6.25 |
47.77 |
8.25 |
37.94 |
10.25 |
30.14 |
|
0.30 |
94.41 |
2.30 |
74.99 |
4.30 |
59.56 |
6.30 |
47.31 |
8.30 |
37.58 |
10.30 |
29.85 |
|
0.35 |
93.50 |
2.35 |
74.27 |
4.35 |
58.99 |
6.35 |
46.86 |
8.35 |
37.22 |
10.35 |
29.57 |
|
0.40 |
92.61 |
2.40 |
73.56 |
4.40 |
58.43 |
6.40 |
46.41 |
8.40 |
36.87 |
10.40 |
29.28 |
|
0.45 |
91.73 |
2.45 |
72.86 |
4.45 |
57.87 |
6.45 |
45.97 |
8.45 |
36.51 |
10.45 |
29.00 |
|
0.50 |
90.85 |
2.50 |
72.16 |
4.50 |
57.32 |
6.50 |
45.53 |
8.50 |
36.17 |
10.50 |
28.73 |
|
0.55 |
89.98 |
2.55 |
71.47 |
4.55 |
56.77 |
6.55 |
45.10 |
8.55 |
35.82 |
10.55 |
28.45 |
|
1.00 |
89.12 |
3.00 |
70.79 |
5.00 |
56.23 |
7.00 |
44.66 |
9.00 |
35.48 |
11.00 |
28.18 |
|
1.05 |
88.27 |
3.05 |
70.12 |
5.05 |
55.69 |
7.05 |
44.24 |
9.05 |
35.14 |
11.05 |
27.91 |
|
1.10 |
87.43 |
3.10 |
69.45 |
5.10 |
55.16 |
7.10 |
43.82 |
9.10 |
34.80 |
11.10 |
27.64 |
|
1.15 |
86.60 |
3.15 |
68.78 |
5.15 |
54.64 |
7.15 |
43.40 |
9.15 |
34.47 |
11.15 |
27.38 |
|
1.20 |
85.77 |
3.20 |
68.13 |
5.20 |
54.11 |
7.20 |
42.98 |
9.20 |
34.14 |
11.20 |
27.12 |
|
1.25 |
84.95 |
3.25 |
67.48 |
5.25 |
53.60 |
7.25 |
42.57 |
9.25 |
33.82 |
11.25 |
26.86 |
|
1.30 |
84.14 |
3.30 |
66.83 |
5.30 |
53.09 |
7.30 |
42.17 |
9.30 |
33.49 |
11.30 |
26.60 |
|
1.35 |
83.33 |
3.35 |
66.19 |
5.35 |
52.58 |
7.35 |
41.76 |
9.35 |
33.17 |
11.35 |
26.35 |
|
1.40 |
82.54 |
3.40 |
66.56 |
5.40 |
52.08 |
7.40 |
41.36 |
9.40 |
32.86 |
11.40 |
26.10 |
|
1.45 |
81.75 |
3.45 |
64.94 |
5.45 |
51.58 |
7.45 |
40.97 |
9.45 |
32.54 |
11.45 |
25.85 |
|
1.50 |
80.97 |
3.50 |
64.32 |
5.50 |
51.09 |
7.50 |
40.58 |
9.50 |
32.23 |
11.50 |
25.60 |
|
1.55 |
80.20 |
3.55 |
63.70 |
5.55 |
50.60 |
7.55 |
40.19 |
9.55 |
31.92 |
11.55 |
25.36 |
|
2.00 |
79.43 |
4.00 |
63.09 |
6.00 |
50.12 |
8.00 |
39.81 |
10.00 |
31.62 |
12.00 |
25.12 |
TABLE 11