Emplariv 4.6 Mg/24 H Transdermal Patch
Out of date information, search anotherC O N F I D E N T I A L
Module 1.3.1.4_Package Leaflet - Procedure version
Package leaflet: Information for the user
EmplaRiv 4.6 mg/24 h transdermal patch Rivastigmine
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you, only. Do not pass it on to others. It may harm them,
even if their signs of illnessare the same as yours.
- If you get any side effects talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
What is in this leaflet:
1. What EmplaRiv is and what it is used for
2. What you need to know before you use EmplaRiv
3. How to use EmplaRiv
4. Possible side effects
5. How to store EmplaRiv
6. Contents of the pack and other information
1. What EmplaRiv is and what it is used for
Rivastigmine belongs to a class of substances called cholinesterase inhibitors. EmplaRiv is used for the treatment of memory disorders in patients with Alzheimer’s disease.
EmplaRiv is indicated in adults.
2. What you need to know before you use EmplaRiv Do not use EmplaRiv
- if you are allergic to rivastigmine or any of the other ingredients of this medicine (listed in section 6)
- if you have ever had an allergic reaction to a similar type of medicine.
- if you have a skin reaction spreading beyond the patch size, if there is a more intense local reaction (such as blisters, increasing skin inflammation, swelling) and if it does not improve within 48 hours after removal of the transdermal patch.
If this applies to you, tell your doctor and do not apply EmplaRiv transdermal patches.
Warnings and precautions
Talk to your doctor or pharmacist before using EmplaRiv
- if you have, or have ever had, an irregular heartbeat.
- if you have, or have ever had, an active stomach or duodenal ulcer.
- if you have, or have ever had, difficulties in passing urine.
- if you have, or have ever had, seizures.
- if you have, or have ever had, asthma or a severe respiratory disease.
- if you suffer from trembling.
Module 1.3.1.4_Package Leaflet - Procedure version
- if you have a low body weight (less than 50 kg).
- if you have gastrointestinal reactions such as feeling sick (nausea), being sick (vomiting) and diarrhoea. You may become dehydrated (losing too much fluid) if vomiting or diarrhoea are prolonged.
- if you have impaired liver function.
If any of these apply to you, your doctor may need to monitor you more closely while you are on this medicine.
If you have not applied a EmplaRiv patch for several days, do not apply the next one before you have talked to your doctor.
The use of EmplaRiv in children and adolescents (age below 18 years) is not recommended.
Other medicines and EmplaRiv
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
EmplaRiv might interfere with anticholinergic medicines (medicines used to relieve stomach cramps or spasms, to treat Parkinson’s disease or to prevent travel sickness)
If you have to undergo surgery whilst using EmplaRiv transdermal patches, tell your doctor that you are using them because they may exaggerate the effects of some muscle relaxants during anaesthesia.
EmplaRiv with food and drink
Food or drink do not affect the use of EmplaRiv transdermal patches because rivastigmine enters the bloodstream through the skin.
Pregnancy and breast-feeding
Tell your doctor if you are pregnant or planning to become pregnant. If you are pregnant, the benefits of using EmplaRiv transdermal patches must be assessed against the possible effects on your unborn child.
You should not breast-feed during treatment with EmplaRiv transdermal patches.
Ask your doctor or pharmacist for advice before using this medicine.
Driving and using machines
Your doctor will tell you whether your illness allows you to drive vehicles and use machines safely. EmplaRiv transdermal patches may cause fainting or severe confusion. If you feel faint or confused do not drive, use machines or perform any other tasks that require your attention.
3. How to use EmplaRiv
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
IMPORTANT: Only wear one EmplaRiv patch at a time. You must remove the previous day’s patch before applying a new one. Do not cut the patch into pieces.
How to start treatment
Module 1.3.1.4_Package Leaflet - Procedure version
Your doctor will tell you which EmplaRiv transdermal patch is most suitable for you.
• Treatment usually starts with EmplaRiv 4.6 mg/24 h.
• The usual daily dose is EmplaRiv 9.5 mg/24 h.
• Only wear one EmplaRiv patch at a time and replace the patch with a new one after 24 hours.
During the course of the treatment your doctor may adjust the dose to suit your individual needs.
If you have not applied a EmplaRiv patch for several days, do not apply the next one before you have talked to your doctor.
Where to apply your EmplaRiv transdermal patch
• Before you apply a patch, make sure that your skin is:
- clean, dry and hairless,
- free of any powder, oil, moisturiser or lotion that could keep the patch from sticking to your skin properly,
- free of cuts, rashes and/or irritations.
■ Carefully remove any existing patch before putting on a new one. Having multiple patches on your body could expose you to an excessive amount of this medicine which could be potentially dangerous.
• Apply only one patch per day to only one of the following locations, as shown in the following diagrams:
- left upper arm or right upper arm
- left upper chest or right upper chest (avoid breast)
- left upper back or right upper back
- left lower back or right lower back
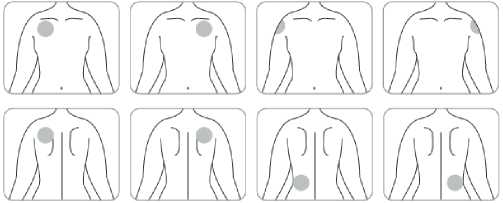
When changing the patch, apply the new one to a different location of skin each time (for example on the right side of your body one day, then on the left side the next day, and on your upper body one day, then on your lower body the next day). Do not apply a new patch to that same location of skin twice within 14 days.
How to apply your EmplaRiv transdermal patch
EmplaRiv patches are thin, translucent, plastic patches that stick to the skin. Each patch is sealed in a sachet that protects it until you are ready to put it on. Do not open the sachet or remove a patch until just before you apply it.
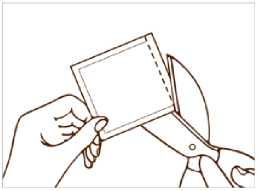
- Each patch is sealed in its own protective sachet. You should only open the sachet when you are ready to apply the patch.
Cut the sachet along the dotted line with scissors and remove the patch from the sachet.
Module 1.3.1.4_Package Leaflet - Procedure version
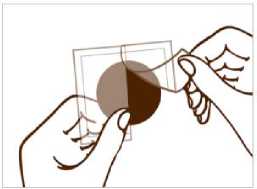
A protective liner covers the sticky side of the patch. Peel off one side of the protective liner and do not touch the sticky part of the patch with the fingers.
Put the sticky side of the patch on the upper or lower back, upper arm or chest and then peel off the second side of the protective liner.
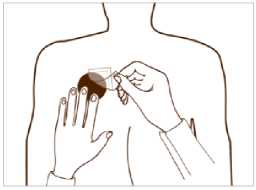
Then press the patch firmly in place with the hand to make sure that the edges stick well.
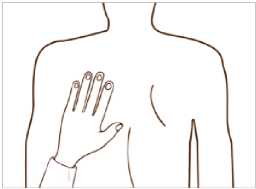
If it helps you, you may write, for example, the day of the week, on the patch with a thin ball point pen.
The patch should be worn continuously until it is time to replace it with a new one. You may wish to experiment with different locations when applying a new patch, to find ones that are most comfortable for you and where clothing will not rub on the patch.
How to remove your EmplaRiv transdermal patch
Gently pull at one edge of the patch to remove it completely from the skin.
How to dispose of your EmplaRiv transdermal patch
After removing a patch, fold it in half with the sticky sides on the inside and press them together.
Module 1.3.1.4_Package Leaflet - Procedure version
Return the used patch to its sachet and dispose of it in such a way that children cannot handle it. Do not touch your eyes with your fingers and wash your hands with soap and water after removing the patch.
If your community burns domestic rubbish, you can dispose of the patch with your domestic rubbish. Otherwise, return used patches to a pharmacy, preferably in the original packaging.
Can you wear your EmplaRiv transdermal patch when you are bathing, swimming, or in the sun?
• Bathing, swimming or showering should not affect the patch. Make sure the patch does not loosen during these activities.
• Do not expose the patch to any external heat sources (e.g. excessive sunlight, saunas, solarium) for long periods of time.
What to do if a patch falls off
If a patch falls off, apply a new one for the rest of the day, then replace it at the same time as usual the next day.
When and for how long to apply your EmplaRiv transdermal patch
• To benefit from treatment, you must apply a new patch every day, preferably at the same time of day.
• Only wear one EmplaRiv patch at a time and replace the patch with a new one after 24 hours.
If you use more EmplaRiv than you should
If you accidentally apply more than one patch, remove all the patches from your skin, then inform your doctor that you have accidentally applied more than one patch. You may require medical attention. Some people who have accidentally taken too much rivastigmine have experienced feeling sick (nausea), being sick (vomiting), diarrhoea, high blood pressure and hallucinations. Slow heart beat and fainting may also occur.
If you forget to use EmplaRiv
If you find you have forgotten to apply a patch, apply one immediately. You may apply the next patch at the usual time the next day. Do not apply two patches to make up for the one that you missed.
If you stop using EmplaRiv
Tell your doctor or pharmacist if you stop using the patch.
If you have not applied a EmplaRiv patch for several days, do not apply the next one before you have talked to your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them.
You may have side effects more often when you start your medicine or when your dose is increased. Usually, the side effects will slowly go away as your body gets used to the medicine.
Take of your patch and tell your doctor straight away, if you notice any of the following side effects which could become serious:
Module 1.3.1.4_Package Leaflet - Procedure version
Uncommon (may affect up to 1 in 100 people)
• Problems with your heartbeat such as slow heartbeat
• Seeing things that are not really there (hallucinations)
• Stomach ulcer
Very rare (may affect up to 1 in 10,000 people)
• Stiff arms or legs
• Trembling hands
Side effects which have been seen since EmplaRiv patch started to be prescribed
• Allergic reaction where the patch was used, such as blisters or inflamed skin
• The signs of Parkinson's disease get worse-such as tremor, stiffness and shuffling
• Inflammation of the pancreas-signs include serious upper stomach pain, often with feeling sick (nausea) or being sick (vomiting)
• Fast or uneven heartbeat
• High blood pressure
• Fits (seizures)
• Falling
• Dehydration (losing too much fluid)
• Liver disorders (yellow skin, yellowing of the whites of eyes, abnormal darkening of the urine or unexplained nausea, vomiting, tiredness and loss of appetite)
• Changes in tests which show how well the liver is working
• Aggression, feeling restless
Take off your patch and tell your doctor straight away, if you notice any of the side effects above.
Other side effects seen with capsules or oral solutions containing rivastigmine and which may occur with the patch:
Very common (may affect more than 1 in 10 people)
• Feeling dizzy
Common (may affect up to 1 in 10 people)
• Too much saliva
• Loss of appetite
• Feeling restless
• Feeling agitated or sleepy
• Generally feeling unwell
• Trembling or feeling confused
• Increased sweating
Uncommon (may affect up to 1 in 100 people)
• Uneven heart rate (e.g. fast heart rate)
• Difficulty sleeping
• Accidental falls
Rare (may affect up to 1 in 1,000 people)
• Fits (seizures)
• Ulcer in the intestine
• Chest pain - this may be caused by heart spasm Very rare (may affect up to 1 in 10,000 people)
Module 1.3.1.4_Package Leaflet - Procedure version
• High blood pressure
• Inflammation of the pancreas-the signs include serious upper stomach pain, often with feeling sick (nausea) or being sick (vomiting)
• Bleeding in the gut - shows as blood in stools or when being sick
• Seeing things that are not there (hallucinations)
• Some people who have been violently sick have had tearing of the tube that connects your mouth with your stomach (oesophagus)
If you get any side effects talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
5. How to store EmplaRiv
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date, which is stated on the carton and sachet after {EXP}. The expiry date refers to the last day of the month.
Store in the original package in order to protect from light.
Keep the transdermal patch in the sachet until use.
This medicinal product does not require any special temperature storage conditions.
Do not use any patch that is damaged or shows signs of tampering.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment (see “How to dispose of your EmplaRiv transdermal patch” above).
6. Content of the pack and other information
What EmplaRiv contains
The active substance is rivastigmine.
Each patch releases 4.6 mg rivastigmine per 24 hours is 5 cm2 and contains 9 mg rivastigmine.
The other ingredients are:
Film: Polyester film
Fluoro-coated polyester film
Drug matrix: Acrylic adhesive, Acrylates copolymer
poly(butyl methacrylat-co-methyl methacrylat)
Adhesive matrix: Silicone adhesive
Printing ink: Black printing ink
What EmplaRiv looks like and contents of the pack
Each transdermal patch is a thin patch consisting of three layers. The outer layer is translucent, white and black-printed with the following:
“Rivastigmine”, “4.6 mg/24 h”
Module 1.3.1.4_Package Leaflet - Procedure version
One transdermal patch is packed in one child-resistant and heat-sealed sachet. The patches are available in packs containing 7, 10, 30, 60 and 90 sachets and in multipacks containing 60 (2 x 30) and 90 (3 x 30) sachets.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder
YES Pharmaceutical Development Services GmbH Bahnstrahe 42 - 46 61381 Friedrichsdorf Germany
Telefon:06172-777447 Fax:06172- 777457 E-Mail:info@yes-services.eu
Manufacturer
PHAST Gesellschaft fur Pharmazeutische Qualitatsstandards mbH Kardinal-Wendel-Strasse 16 66424 Homburg Germany
Telefon:06841 984890 Fax:06841 9848950 E-Mail: info@phast.de
ACC GmbH Analytical Clinical Concepts Schontalweg 9 63849 Leidersbach Germany
Telefon:06028 4043 0 Fax:06028 4043 20 E-Mail: mail@accgmbh.com
Laboratoires Plasto Sante 42 rue de Longvic 21300 CHENOVE France
This medicinal product is authorised in the Member States of the EEA under the following names:
Germany EmplaRiv 4,6 mg/24 Stunden transdermales Pflaster
United Kingdom EmplaRiv 4.6 mg/24 h Transdermal Patch
Module 1.3.1.4_Package Leaflet - Procedure version
This leaflet was last revised in 07/2013
July2013 Version 02 Mod. 1.3.1.4, Page 9