Equanox
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Equanox
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Nitrous Oxide Ph. Eur. 50% and Oxygen Ph. Eur. 50%
3 PHARMACEUTICAL FORM
Inhalation gas
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
As an analgesic where rapid-onset powerful short-term relief of pain is required.
4.2 Posology and method of administration
Use in adults, including the elderly and children
For respiratory use
Equanox is administered through a facemask or mouthpiece. The mask is connected to an Equanox supply through a demand valve system. The valve is operated by the act of inhalation of the patient and closes down when the patent ceases to inhale.
In nearly all cases, Equanox is self-administered, but it may be administered by attendant medical personnel trained in its use. Since pain is usually relieved by a concentration of 25% nitrous oxide, continued inhalation does not occur. However, should inhalation continue, light anaesthesia results and inhalation ceases as the mask falls away.
Equanox should not be used for more than a total of 24 hours, or more frequently than every 4 days, without close clinical supervision and haematological monitoring (see sections 4.4 and 4.8)
Instructions for Use and Handling of Equanox cylinders
If the cylinders of Equanox have not been stored horizontally for at least 24 hours at a temperature above 10°C, (but not exceeding 38°C), then, before use, the cylinders should be maintained at a temperature of above 10°C for at least 2 hours and then completely inverted 3 times. Alternatively, cylinders may be placed in warm water at body temperature for 5 minutes and then completely inverted 3 times.
Thorough ventilation or scavenging of waste gases should take place to reduce operating theatre and equivalent treatment room levels of ambient nitrous oxide to a level below 100ppm.
Preparation _ for use
1. Cylinder valves should be opened momentarily prior to use to blow any foreign matter out of the outlet.
2. Ensure that the connecting face on the yoke, manifold or regulator is clean and the sealing washer or ‘O’ ring where fitted is in good condition.
3. Cylinder valves must be opened slowly.
4. Only the appropriate regulator should be used for the particular gas concerned.
5. Pipelines for medical gases should be installed in accordance with the conditions set out in HTM 02.
6. Cylinder valves and any associated equipment must never be lubricated and must be kept free from oil and grease.
Leaks
1. Should leaks occur this will usually be evident by a hissing noise.
2. Leaks can be found by brushing the suspected area with an approved leak test solution.
3. There are no user serviceable parts associated with these valves, do not attempt to correct any problems with leakage from any part of the valve itself. Label any faulty containers, and return them to Air Liquide for repair.
4. Sealing or jointing compounds must never be used to cure a leak.
5. Never use excessive force when connecting equipment to cylinders.
Use of Cylinders
1. Cylinders should be handled with care and not knocked violently or allowed to fall.
2. Cylinders should only be moved with the appropriate size and type of trolley.
3. When in use cylinders should be firmly secured to a suitable cylinder support.
4. Cylinders containing liquefiable gas must always be used vertically with the valve uppermost.
5. Medical gases must only be used for medicinal purposes.
6. Smoking and naked lights must not be allowed within the vicinity of cylinders or pipeline outlets.
7. After use cylinder valves should be closed using moderate force only and the pressure in the regulator or tailpipe released.
8. When only a small amount of gas remains in a cylinder, the cylinder valve must be closed. It is important to leave a small residual pressure in each cylinder after use, in order to protect the inside of the cylinder from contamination.
4.3 Contraindications
Nitrous oxide should not be used with any condition where air is entrapped within the body and where its expansion might be dangerous.
4.4 Special warnings and precautions for use
The nitrous oxide constituent of Equanox causes inactivation of vitamin B12 (a co-factor of methionine synthase) which interferes with folate metabolism. Thus DNA synthesis is impaired following prolonged nitrous oxide administration. Prolonged or frequent use of nitrous oxide may result in megaloblastic bone marrow changes and possibly myeloneuropathy and subacute combined degeneration of the spinal cord (see also 4.8).
Equanox should not be used for more than a total of 24 hours, or more frequently than every 4 days, without close clinical supervision and haematological monitoring. Specialist advice should be sought from a haematologist in such cases. Haematological assessment should include an assessment for megaloblastic change in red blood cells and hypersegmentation of neutrophils. Neurological toxicity can occur without anaemia or macrocytosis and with B12 levels in the normal range.
In patients with undiagnosed subclinical deficiency of vitamin B12 neurological toxicity has occurred after single exposures to nitrous oxide during general anaesthesia.
Thorough ventilation or scavenging of waste gases should take place to reduce operating theatre and equivalent treatment room levels of ambient nitrous oxide to a level below 100ppm.
4.5 Interaction with other medicinal products and other forms of interaction
The nitrous oxide constituent of Equanox causes inactivation of vitamin B12
4.6 Pregnancy and lactation
Mild skeletal teratogenic changes have been observed in pregnant rat embryos when the dam has been exposed to high concentrations of nitrous oxide during the period of organogenesis.
However, no increased incidence of foetal malformation has been discovered in various epidemiological studies and case reports in human beings.
There is no published material which shows that nitrous oxide is toxic to the human foetus. Therefore, there is no absolute contra-indication to its use in the first 16 weeks of pregnancy.
Equanox can be used during lactation.
The current UK work place exposure limit (WEL) for nitrous oxide is 100ppm (8 hour time weighted average reference period). This occupational exposure level is sufficient to protect against any potential adverse reproductive effect of exposure to nitrous oxide in an occupational setting.
4.7 Effects on ability to drive and use machines
The nitrous oxide constituent of Equanox is rapidly eliminated from the body and the effects will normally cease shortly after administration has stopped.
When Equanox is used as a sole analgesic/sedative agent, driving, the use of machinery
and other psycho-motor activities is not recommended until:
• the healthcare professional has judged that the patient has returned to their normal mental state
• the patient feels that they are competent to drive after the relevant procedure is completed
• at least 30 minutes has elapsed after the administration of Equanox has ceased. Additional precaution is needed when Equanox is administered with concomitant medication, and this should be considered prior to any patient being permitted to drive,
operate machinery and undertake other psycho-motor activities.
4.8 Undesirable effects
Events such as euphoria, disorientation, sedation, nausea, vomiting, dizziness and generalised tingling are commonly described. These events are generally minor and rapidly reversible.
Prolonged or frequent use of nitrous oxide, including heavy occupational exposure and addiction, may result in megalobastic anaemia. Agranulocytosis has been reported following prolonged nitrous oxide administration (see section 4.4).
Myeloneuropathy and sub acute combined degeneration, have also been reported following prolonged or frequent use. However in patients with undiagnosed sub-clinical deficiency of vitamin B12, neurological toxicity has occurred after a single exposure to nitrous oxide for anaesthesia (see section 4.4).
Theoretically similar adverse results could result from heavy and prolonged exposure to Equanox.
The nitrous oxide constituent of Equanox passes into all gas containing spaces in the body faster than nitrogen passes out. Prolonged exposure to Equanox may result in bowel distension, middle ear damage and rupture of ear drums. Addiction to a 50% nitrous oxide/50% oxygen gas mixture has been reported.
4.9 Overdose
In normal medical use there is no risk of overdose with Equanox as, with continued inhalation, light anaesthesia results and inhalation ceases as the face mask falls away.
Excessive inhalation of Equanox will ultimately result in unconsciousness, passing through stages of increasing lightheadedness and intoxication. The treatment is removal to fresh air, mouth-to-mouth resuscitation and, if necessary, the use of an oxygen resuscitator.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Nitrous oxide is a colourless, odourless gas with molecular weight 44.01, a boiling point of -88.6oC (at 1 bar) and a density of 1.875 kg/m3 (at 15°C and 1013mb).
Nitrous oxide is not very soluble in water but is fifteen times more soluble than oxygen. Water dissolves nitrous oxide, taking 100 vol % and blood plasma 45 vol %.
Nitrous oxide is a potent analgesic and a weak anaesthetic. Induction with nitrous oxide is relatively rapid, but a concentration of about 70% is needed to produce unconsciousness. Endorphins are probably involved in the analgesic effect; a concentration of 25% nitrous oxide is usually adequate to provide a marked reduction in pain.
Oxygen is a colourless, odourless gas with molecular weight 32, a boiling point of -183.1°C (at 1 bar) and a density of 1.335 kg/m3 (at 15°C and 1013mb).
Oxygen is present in the atmosphere at 21% and is essential for life.
At the concentrations in Equanox, oxygen has no discernible pharmaceutical effect other than the beneficial effects of an oxygen enriched mixture in certain cases.
Equanox gas mixture has a specific gravity of 1.319 (at 15°C) and a density of 1.615 kg/m3 (at 15°C and 1013mb).
5.2 Pharmacokinetic properties
Nitrous oxide is a low potency inhalation anaesthetic and high potency analgesic.
At a constant inspired concentration, the rise time of alveolar concentrations is faster than that of any other anaesthetic agent. The elimination of nitrous oxide equally is faster than that of any other anaesthetic. This characteristic is especially valuable in analgesia for short term pain.
Nitrous oxide is eliminated unchanged from the body mostly by the lungs. There are no essential observations about the pharmacokinetics of oxygen at this concentration.
5.3 Preclinical safety data
There is no additional data of relevance to the prescriber.
6.1 List of excipients
None
6.2 Incompatibilities
There are no major incompatibilities with Equanox.
6.3 Shelf life
Five years.
6.4 Special precautions for storage
Cylinders should be kept out of the reach of children.
Equanox is non-flammable but strongly supports combustion (including some materials which do not normally burn in air). It is highly dangerous when in contact with oils, greases, tarry substances and many plastics due to the risk of spontaneous combustion with high pressure gases.
Nitrous oxide begins to separate out from Equanox if the temperature falls below about -6oC. A homogenous mixture is again obtained when the temperature is raised to above 10oC and the cylinder agitated. Before use, to ensure it is properly mixed, cylinders should be stored, horizontally for 24 hours at a temperature above 10 C but not exceeding 38 C.
The normal precautions required in the storage of medical gas cylinders as described below are applicable.
• Cylinders should be stored under cover, preferably inside, kept dry and clean and not subjected to extremes of heat or cold.
• Cylinders should not be stored near stocks of combustible materials or near sources of heat.
• Warning notices prohibiting smoking and naked lights must be posted clearly.
• Emergency services should be advised of the location of the cylinder store.
• Medical cylinders containing different gases should be segregated and identified within the store.
• Full and used cylinders should be stored separately. Full cylinders should be used in strict rotation.
• Cylinders must not be repainted, have any markings obscured or labels removed.
• F size cylinders and larger should be stored vertically E size cylinders and smaller should be stored horizontally.
• Precautions should be taken to protect cylinders from theft.
Equanox is supplied in a gas cylinder, with valve, suitable for the pressure required for the product.
The types of cylinders normally used are specified in the following table.
|
Cylinder Size |
Water Volume (litres) |
Fill Pressure (bar) |
Fill Volume (m3) |
Valve Type(1) |
|
CC |
1.0 |
137 |
0.21 |
Integral pressure regulator valve with flow control and Schraeder connection |
|
CC 200 |
1.0 |
200 |
0.29 |
Integral pressure regulator valve with flow control and Schraeder connection |
|
AD |
2.0 |
137 |
0.42 |
Integral pressure regulator valve with flow control and Schraeder connection |
|
AD 170 |
2.0 |
170 |
0.50 |
Integral pressure regulator valve with flow control and Schraeder connection |
|
AD 200 |
2.0 |
200 |
0.58 |
Integral pressure regulator valve with flow control and Schraeder connection |
|
CD |
2.0 |
137 |
0.42 |
Integral pressure regulator valve with flow control and Schraeder connection |
|
CD 200 |
2.0 |
200 |
0.58 |
Integral pressure regulator valve with flow control and Schraeder connection |
|
D |
2.32 |
137 |
0.50 |
Pin-index |
|
F |
9.43 |
137 |
2.00 |
Pin-index |
|
F4 |
9.43 |
137 |
2.00 |
Integral pressure regulator valve with Schraeder connection |
|
F4 170 |
9.43 |
170 |
2.40 |
Integral pressure regulator valve with Schraeder connection |
|
F4 200 |
9.43 |
200 |
2.73 |
Integral pressure regulator valve with Schraeder connection |
|
AF4 |
10.0 |
137 |
2.12 |
Integral pressure regulator valve with Schraeder connection |
|
AF4 200 |
10.0 |
200 |
2.90 |
Integral pressure regulator valve with Schraeder connection |
|
G |
23.6 |
137 |
5.00 |
Pin-index |
|
J |
50.0 |
137 |
10.60 |
Pin-index |
|
J 200 |
50.0 |
200 |
14.50 |
Pin-index |
Note: (1) Cylinder valves conform to appropriate international standards, BS EN ISO 407:2004, for pin-index valves, the integral pressure regulator valves are CE marked devices with 4 bar regulated outlets, the Schraeder connector conforms to BS 5682.
The colour of Equanox cylinders in the UK is in a period of change.
The colour coding of the shoulder of Equanox is blue and white quarters. The body of the cylinder will be either blue or white.
The aim is to complete a period of change over from the blue body to the white bodied cylinder. The shoulder colour of the cylinder will remain as blue and white quarters. This period of change will be completed by January 1st 2026.
The images below represent the new and current colour coding of Equanox cylinders:
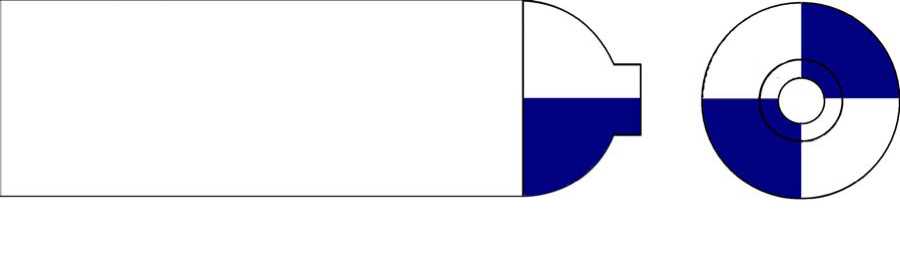
New white bodied Equanox cylinder colour coding
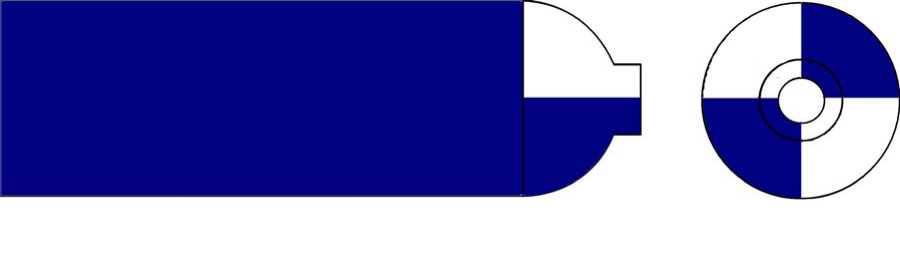
Current Equanox cylinder colour coding
6.6 Special precautions for disposal
Immediately return used cylinders to the used cylinder store for return to Air Liquide.
7 MARKETING AUTHORISATION HOLDER
Air Liquide Limited Station Road Coleshill Birmingham West Midlands B46 1JY
8 MARKETING AUTHORISATION NUMBER
PL 15929/0008
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
04/02/1998 / 17/03/2003
10 DATE OF REVISION OF THE TEXT
19/08/2014