Escitalopram 20 Mg Film-Coated Tablets
Out of date information, search another|
TEVA UK Ref: 231-30-86398-D LEA ESCITALOPRAM A/S FC TAB TUK <KRAKOW |
Version: 2 26 March 2014 |
TEVA UK Ref: 231-30-86398-D LEA ESCITALOPRAM A/S FC TAB TUK <KRAKOW |
Version: 2 26 March 2014 |
PACKAGE LEAFLET: INFORMATION FOR THE USER
ESCITALOPRAM 5 mg, 10 mg, 15 mg AND 20 mg FILM-COATED TABLETS
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Escitalopram is and what it is used for
2. What you need to know before you take Escitalopram
3. How to take Escitalopram
4. Possible side effects
5. How to store Escitalopram
6. Contents of the pack and other information
OH What Escitalopram is and what it is used for
Escitalopram belongs to a group of antidepressants called selective serotonin reuptake inhibitors (SSRIs). These medicines act on the serotonin-system in the brain by increasing the serotonin level. Disturbances in the serotonin-system are considered an important factor in the development of depression and related diseases.
Escitalopram Film-coated Tablets contain escitalopram and are used to treat depression (major depressive episodes) and anxiety disorders (such as panic disorder with or without agoraphobia, social anxiety disorder, generalised anxiety disorder and obsessive-compulsive disorder).
^ What you need to know
before you take Escitalopram
Do not take Escitalopram
if you are allergic (hypersensitive) to escitalopram or any of the other ingredients of Escitalopram Film-coated Tablets (see section 6 "Contents of the pack and other information")
if you take other medicines that belong to a group called MAO inhibitors, including selegiline (used in the treatment of Parkinson's disease), moclobemide (used in the treatment of depression) and linezolid (an antibiotic)
if you are born with or have had an episode of abnormal heart rhythm (seen at ECG; an examination to evaluate how the heart is functioning)
if you take medicines for heart rhythm problems or that may affect the heart's rhythm (see section 2 "Other medicines and Escitalopram").
Warnings and precautions
Talk to your doctor or pharmacist before taking Escitalopram: if you have epilepsy. Treatment with Escitalopram should be stopped if seizures occur for the first time or if there is an increase in the seizure frequency (see also section 4 "Possible side effects") if you suffer from impaired liver or kidney function. Your doctor may need to adjust your dosage if you have diabetes. Treatment with Escitalopram may alter glycaemic control. Insulin and/or oral hypoglycaemic dosage may need to be adjusted if you have a decreased level of sodium in the blood if you have a tendency to easily develop bleedings or bruises if you are receiving electroconvulsive treatment if you have coronary heart disease if you suffer or have suffered from heart problems or have recently had a heart attack if you have a low resting heart-rate and/or you know that you may have salt depletion as a result of prolonged severe diarrhoea and vomiting (being sick) or usage of diuretics (water tablets) if you experience a fast or irregular heartbeat, fainting, collapse or dizziness on standing up, which may indicate abnormal functioning of the heart rate
if you have glaucoma (increased eye pressure).
Please note
Some patients with manic-depressive ness may enter into a manic phase. This is characterised by unusual and rapidly changing ideas, inappropriate happiness and excessive physical activity. If you experience this, contact your doctor.
Symptoms such as restlessness or difficulty to sit or stand still can also occur during the first weeks of the treatment. Tell your doctor mmediately if you experience these symptoms.
Thoughts of suicide and worsening of your depression or anxiety disorder
If you are depressed and/or have anxiety disorders you can sometimes have thoughts of harming or killing yourself. These may be increased when first starting antidepressants, since these medicines all take time to work, usually about two weeks but sometimes longer.
You may be more likely to think like this:
if you have previously had thoughts about killing or harming yourself
if you are a young adult.
Information from clinical trials has shown an increased risk of suicidal behaviour in adults aged less than 25 years with psychiatric conditions who were treated with an antidepressant.
If you have thoughts of harming or killing yourself at any time, contact your doctor or go to a hospital straight away.
You may find it helpful to tell a relative or close friend that you are depressed or have an anxiety disorder and ask them to read this leaflet. You might ask them to tell you if they think your depression or anxiety is getting worse, or if they are worried about changes in your behaviour.
Children and adolescents under 18 years of age
Escitalopram should normally not be used for children and adolescents under 18 years. Also, you should know that patients under 18 have an ncreased risk of side effects such as suicide attempts, suicidal thoughts and hostility (predominately aggression, oppositional behaviour and anger) when they take this class of medicines. Despite this, your doctor may prescribe Escitalopram for patients under 18 because he/she decides that this is in their best nterest. If your doctor has prescribed Escitalopram for a patient under 18 and you want to discuss this, please go back to your doctor. You should nform your doctor if any symptoms listed above develop or worsen when patients under 18 are taking Escitalopram. Also, the long term safety effects concerning growth, maturation and cognitive and behavioural development of Escitalopram in this age group have not yet been demonstrated.
Other medicines and Escitalopram
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicine.
Tell your doctor if you are taking any of the following medicines:
• “non-selective monoamine oxidase inhibitors (MAOIs)" containing phenelzine, iproniazid, isocarboxazid, nialamide and tranylcypromine as active ingredients. If you have taken any of these medicines you will need to wait 14 days before you start taking Escitalopram. After stopping Escitalopram you must allow 7 days before taking any of these medicines
• “reversible, selective MAO-A inhibitors" containing moclobemide (used to treat depression)
• “irreversible mAO-B inhibitors" containing selegiline (used to treat Parkinson's disease). These increase the risk of side effects
• the antibiotic linezolid
• lithium (used in the treatment of manic-depressive disorder) and tryptophan
• imipramine and desipramine (both used to treat depression)
• sumatriptan and similar medicines (used to treat migraine) and tramadol (used against severe pain). These increase the risk of a rare but potentially serious side effect known as serotonin syndrome
• cimetidine, omeprazole, esomeprazole, lansoprazole (used to treat stomach ulcers), fluvoxamine (antidepressant) and ticlopidine (used to reduce the risk of stroke). These may cause increased blood levels of Escitalopram
• St. John's Wort (hypericum perforatum) - a herbal remedy used for depression
• acetylsalicylic acid and non-steroidal anti-inflammatory drugs (medicines used for pain relief or to thin the blood, so called anti-coagulants). These may increase bleeding-tendency
• warfarin, dipyridamole, acenocumarol and phenprocoumon (medicines used to thin the blood, so called anti-coagulants). Your doctor will probably check the coagulation time of your blood when starting and discontinuing Escitalopram in order to verify that your dose of anti-coagulant is still adequate
• mefloquin (used to treat malaria), bupropion (used to treat depression) and tramadol (used to treat severe pain) due to a possible risk of a lowered threshold for seizures
• neuroleptics/antipsychotics (medicines to treat schizophrenia, psychosis) due to a possible risk of a lowered threshold for seizures, and antidepressants
• flecainide, propafenone and metoprolol (used in cardio-vascular diseases) clomipramine and nortriptyline (antidepressants) and risperidone, thioridazine and haloperidol (antipsychotics). The dosage of Escitalopram may need to be adjusted
• any drug which can decrease levels of potassium (hypokalaemia) or magnesium (hypomagnesaemia) in blood.
Do not take Escitalopram if you take medicines for heart rhythm problems or medicines that may affect the heart's rhythm, such as Class IA and III antiarrhythmics, antipsychotics (e.g. phenothiazine derivatives, pimozide, haloperidol), tricyclic antidepressants, certain antimicrobial agents (e.g. sparfloxacin, moxifloxacin, erythromycin IV, pentamidine, anti-malarian treatment particularly halofantrine), certain antihistamines (astemizole, mizolastine). If you have any further questions about this you should speak to your doctor.
Escitalopram with food, drink and alcohol
Escitalopram can be taken with or without food (see section 3 "How to take Escitalopram").
As with many medicines, combining Escitalopram with alcohol is not advisable, although Escitalopram is not expected to interact with alcohol.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine. Do not take Escitalopram if you are pregnant or breast-feeding unless you and your doctor have discussed the risks and benefits involved.
Citalopram, a medicine like escitalopram, has been shown to reduce the quality of sperm in animal studies. Theoretically, this could affect fertility, but impact on human fertility has not been observed as yet.
If you take Escitalopram during the last 3 months of your pregnancy you should be aware that the following effects may be seen in your newborn baby: trouble with breathing, bluish skin, fits, body temperature changes, feeding difficulties, vomiting, low blood sugar, stiff or floppy muscles, vivid reflexes, tremor, jitteriness, irritability, lethargy, constant crying, sleepiness and sleeping difficulties. If your newborn baby has any of these symptoms, please contact your doctor immediately.
Make sure your midwife and/or doctor know you are on Escitalopram. When taken during pregnancy, particularly in the last 3 months of pregnancy, medicines like Escitalopram may increase the risk of a serious condition in babies, called persistent pulmonary hypertension of the newborn (PPHN), making the baby breathe faster and appear bluish. These symptoms usually begin during the first 24 hours after the baby is born. If this happens to your baby you should contact your midwife and/or doctor immediately.
If used during pregnancy Escitalopram should never be stopped abruptly.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
You are advised not to drive a car or operate machinery until you know how Escitalopram affects you.
How to take Escitalopram
Always take Escitalopram exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
Adults
Depression
The normally recommended dose of Escitalopram is 10 mg taken as one daily dose. The dose may be increased by your doctor to a maximum of 20 mg per day.
Panic disorder
The starting dose of Escitalopram is 5 mg as one daily dose for the first week before increasing the dose to 10 mg per day. The dose may be further increased by your doctor to a maximum of 20 mg per day.
Social anxiety disorder
The normally recommended dose of Escitalopram is 10 mg taken as one daily dose. Your doctor can either decrease your dose to 5 mg per day or increase the dose to a maximum of 20 mg per day, depending on how you respond to the medicine.
Generalised anxiety disorder
The normally recommended dose of Escitalopram is 10 mg taken as one daily dose. The dose may be increased by your doctor to a maximum of 20 mg per day.
Obsessive-compulsive disorder
The normally recommended dose of Escitalopram is 10 mg taken as one daily dose. The dose may be increased by your doctor to a maximum of 20 mg per day.
Older people (above 65 years of age)
The recommended starting dose of Escitalopram is 5 mg taken as one daily dose. The dose may be increased by your doctor to 10 mg per day.
Use in children and adolescents (below 18 years of age)
Escitalopram should not normally be given to children and adolescents. For further information please see section 2 “What you need to know before you take Escitalopram'.'
You can take Escitalopram with or without food. Swallow the tablet with some water. Do not chew them, as the taste is bitter.
Escitalopram 10 mg Film-coated Tablets, Escitalopram 20 mg Film-coated Tablets
If necessary, you can divide the tablets by firstly placing the tablet on a flat surface with the score facing upwards. The tablets may then be broken by pressing down on each end of the tablet, using both forefingers as shown in the drawing.
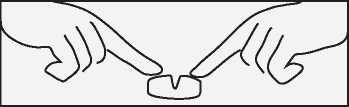
Duration of treatment
It may take a couple of weeks before you start to feel better. Continue to take Escitalopram even if it takes some time before you feel any improvement in your condition.
Do not change the dose of your medicine without talking to your doctor first.
Continue to take Escitalopram for as long as your doctor recommends. If you stop your treatment too soon, your symptoms may return. It is recommended that treatment should be continued for at least 6 months after you feel well again.
If you take more Escitalopram than you should
If you take more than the prescribed dose of Escitalopram, contact your doctor or nearest hospital emergency department immediately. Do this even if there are no signs of discomfort. Some of the signs of an overdose could be dizziness, tremor, agitation, convulsion, coma, nausea, vomiting, change in heart rhythm, decreased blood pressure and change in body fluid/salt balance.
Take the Escitalopram box/container with you when you go to the doctor or hospital.
If you forget to take Escitalopram
Do not take a double dose to make up for forgotten doses. If you do forget to take a dose and you remember before you go to bed, take it straight away. Carry on as usual the next day. If you only remember during the night, or the next day, leave out the missed dose and carry on as usual.
If you stop taking Escitalopram
Do not stop taking Escitalopram until your doctor tells you to do so. When you have completed your course of treatment, it is generally advised that the dose of Escitalopram is gradually reduced over a number of weeks.
When you stop taking Escitalopram, especially if it is abruptly, you may feel discontinuation symptoms. These are common when treatment with Escitalopram is stopped. The risk is higher, when Escitalopram has been used for a long time or in high doses or when the dose is reduced too quickly. Most people find that the symptoms are mild and go away on their own within two weeks.
However, in some patients they may be severe in intensity or they may be prolonged (2-3 months or more). If you get severe discontinuation symptoms when you stop taking Escitalopram, please contact your doctor. He or she may ask you to start taking your tablets again and come off them more slowly.
Discontinuation symptoms include: Feeling dizzy (unsteady or off-balance), feelings like pins and needles, burning sensations and (less commonly) electric shock sensations, including in the head, sleep disturbances (vivid dreams, nightmares, inability to sleep), feeling anxious, headaches, feeling sick (nausea), sweating (including night sweats), feeling restless or agitated, tremor (shakiness), feeling confused or disorientated, feeling emotional or irritable, diarrhoea (loose stools), visual disturbances, fluttering or pounding heartbeat (palpitations).
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Possible side effects
Like all medicines, Escitalopram can cause side effects, although not everybody gets them.
The side effects usually disappear after a few weeks of treatment.
Please be aware that many of the effects may also be symptoms of your illness and therefore will mprove when you start to get better.
If you experience the following side effects you should contact your doctor or go to the hospital straight away:
Uncommon (may affect up to 1 in 100 people):
unusual bleeds, including gastrointestinal bleeds.
Rare (may affect up to 1 in 1,000 people):
if you experience swelling of skin, tongue, lips, or face, or have difficulties breathing or swallowing (allergic reaction), contact your doctor or go to a hospital straight away
if you have a headache, increased heart rate, shivering, high fever, sweating, agitation, confusion, nausea or diarrhoea, trembling and abrupt contractions of muscles these may be signs of a rare but potentially life-threatening condition called serotonin syndrome. If you feel like this contact your doctor.
Some patients have reported
(frequency can not be estimated from the available data) fast, irregular heart beat, fainting which could be symptoms of a life-threatening condition known as Torsades de Pointes difficulties urinating seizures (fits), see also section “Warnings and precautions" yellowing of the skin and the white in the eyes are signs of liver function impairment/hepatitis.
In addition to above the following side effects have been reported:
Very common (may affect more than 1 in 10 people): feeling sick (nausea) headache.
Common (may affect up to 1 in 10 people):
blocked or runny nose (sinusitis) decreased or increased appetite anxiety, restlessness, abnormal dreams, difficulties falling asleep, feeling sleepy, dizziness, yawning, tremors, prickling of the skin diarrhoea, constipation, vomiting, dry mouth increased sweating pain in muscle and joints (arthralgia and myalgia) sexual disturbances (delayed ejaculation, problems with erection, decreased sexual drive and women may experience difficulties achieving orgasm) fatigue, fever increased weight.
Uncommon (may affect up to 1 in 100 people):
• nettle rash (urticaria), rash, itching (pruritus)
• grinding one's teeth, agitation, nervousness, panic attack, confusion state
• disturbed sleep, taste disturbance, fainting (syncope)
• enlarged pupils (mydriasis), visual disturbance, ringing in the ears (tinnitus)
• loss of hair
• vaginal bleeding at irregular intervals, particularly between the expected menstrual periods or an abnormally heavy and prolonged menstrual period at regular intervals
• decreased weight
• fast heart beat
• swelling of the arms or legs (oedema)
• nosebleeds.
Rare (may affect up to 1 in 1,000 people):
• aggression, depersonalisation, hallucination
• slow heart beat.
Some patients have reported
(frequency can not be estimated from the available data):
• thoughts of harming yourself or thoughts of killing yourself, see also section "Take special care with Escitalopram"
• decreased levels of sodium in the blood (the symptoms are feeling sick and unwell with weak muscles or confused)
• dizziness when you stand up due to low blood pressure (orthostatic hypotension)
• abnormal liver function test (increased amounts of liver enzymes in the blood)
• movement disorders (involuntary movements of the muscles)
• painful erections (priapism)
• bleeding disorders including skin and mucous bleeding (ecchymosis) and low level of blood platelets (thrombocytopenia)
• sudden swelling of skin or mucosa (angioedemas)
• increase in the amount of urine excreted (inappropriate ADH secretion)
• flow of milk in women that are not nursing
• mania
• an increased risk of bone fractures has been observed in patients taking this type of medicine
• alteration of the heart rhythm (called "prolongation of QT interval" seen on ECG, electrical activity of the heart).
In addition, a number of side effects are known to occur with drugs that work in a similar way to escitalopram (the active ingredient of Escitalopram Film-coated Tablets). These are:
• motor restlessness (akathisia)
• anorexia.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via the Yellow Card Scheme at:
www.mhra.gov.uk/yellowcard By reporting side effects you can help provide more information on the safety of this medicine.
How to store Escitalopram
Keep this medicine out of the sight and reach of children.
Store below 30°C. Store in the original package (carton) in order to protect from light and moisture.
Do not use the drug after the expiry date which is printed on the blister and carton after the abbreviation EXP. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer needed.
These measures will help to protect the environment.
©Contents of the pack and other information
What Escitalopram contains
The active substance is escitalopram. Each Escitalopram Film-coated Tablet contains 5 mg, 10 mg, 15 mg or 20 mg escitalopram (as oxalate).
• The other ingredients are:
Core: microcrystalline cellulose, colloidal silica anhydrous, croscarmellose sodium, stearic acid and magnesium stearate.
Coating: hypromellose (E464), macrogol 400, and titanium dioxide (E171).
What Escitalopram looks like and contents of the pack
Escitalopram 5 mg is a white, film-coated tablet, marked "93" on one side and "7414" on the other.
Escitalopram 10 mg is a white, film-coated tablet, scored on one side and marked "9" on one side and "3" on the other. The other side of the tablet is marked "7462" The tablet can be divided into equal halves.
Escitalopram 15 mg is a white, film-coated tablet, scored on one side and marked "S" on one side and "C" on the other. The other side of the tablet is marked "15"
The score line is only to facilitate breaking for ease of swallowing and not to divide into equal doses.
Escitalopram 20 mg is a white, film-coated tablet, scored on one side and marked "9" on one side and "3" on the other. The other side of the tablet is marked "7463"
The tablet can be divided into equal halves.
Escitalopram comes in blister packs of 7, 10, 14, 20, 28, 30, 49, 50, 56, 60, 90, 98, 100, 112, 120, 200 and 500 film-coated tablets and perforated unit dose blister 49x1,50x1, 100x1 and 500x1 film-coated tablets. PVC/PVdC-Aluminium blisters are in the carton.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder
Teva UK Limited, Eastbourne, BN22 9AG, UK.
Manufacturer
Teva Operations Poland Sp. Z.0.0. 80 Mogilska Str., 31-546 Krakow, Poland.
This leaflet was last revised in 03/2014
PL 00289/1724 PL 00289/1725 PL 00289/1726 PL 00289/1727
86398-D
|
TTWil |
Ref: 231-30-91411-B LEA ESCITALOPRAM A/S TAB TUK |
04 February 2014 | |
|
TEVA UK LIMITED |
| Version: 10 |
Trackwise Parent: N/A Child: N/A | |
|
PL Number(s), MA Holder & Packer: |
PL 00289/1724-7. TEVA UK Limited Licence (Responsible Regulatory Officer = New Products Team), Packed at TEVA UK Limited, Eastbourne. Packing line: UHLMANN 1030. | ||
F. P. Code: 231-10-04981
231-10-04983 231-10-04985
Dimensions:
PANTONE® GREEN C
(PANTONE® is a registered
Pharma Code: Third party code: Fonts
Base Font Size:
868 (101100101)
N/A
Univers
Times (roman numerals only) 9 Pt
L:
W:
Folds to:
323 mm 320 mm 160 mm
BLACK
Please refer to the latest version of the full base material specification: 92653, 92655, 92651
IMPORTANT: Artwork, text and content must not be reset, remade, amended or altered. The only exceptions to this are: bleeds, chokes, spreads or other print related adjustments required for reproduction by the supplier. We must receive a copy of any 3rd Party Supplier’s Proof before approval to print will be granted.
PAGE 4: AFTER FOLDING REAR FACE (OUTSIDE OF REEL AFTER FOLDING AND TACKING)
FOLD
LINE.
PAGE 1: AFTER FOLDING FRONT FACE (INSIDE OF REEL AFTER FOLDING AND TACKING)
Uncommon (may affect up to 1 in 100 people):
• nettle rash (urticaria), rash, itching (pruritus)
• grinding one's teeth, agitation, nervousness, panic attack, confusion state
• disturbed sleep, taste disturbance, fainting (syncope)
• enlarged pupils (mydriasis), visual disturbance, ringing in the ears (tinnitus)
• loss of hair
• vaginal bleeding at irregular intervals, particularly between the expected menstrual periods or an abnormally heavy and prolonged menstrual period at regular intervals
• decreased weight
• fast heart beat
• swelling of the arms or legs (oedema)
• nosebleeds.
Rare (may affect up to 1 in 1,000 people):
• aggression, depersonalisation, hallucination
• slow heart beat.
Some patients have reported (frequency can not be estimated from the available data):
• thoughts of harming yourself or thoughts of killing yourself, see also section "Take special care with Escitalopram"
• decreased levels of sodium in the blood (the symptoms are feeling sick and unwell with weak muscles or confused)
• dizziness when you stand up due to low blood pressure (orthostatic hypotension)
• abnormal liver function test (increased amounts of liver enzymes in the blood)
• movement disorders (involuntary movements of the muscles)
• painful erections (priapism)
• bleeding disorders including skin and mucous bleeding (ecchymosis) and low level of blood platelets (thrombocytopenia)
• sudden swelling of skin or mucosa (angioedemas)
• increase in the amount of urine excreted (inappropriate ADH secretion)
• flow of milk in women that are not nursing
• mania
• an increased risk of bone fractures has been observed in patients taking this type of medicine
• alteration of the heart rhythm (called "prolongation of QT interval" seen on ECG, electrical activity of the heart).
In addition, a number of side effects are known to occur with drugs that work in a similar way to escitalopram (the active ingredient of Escitalopram Film-coated Tablets). These are:
• motor restlessness (akathisia)
• anorexia.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard By reporting side effects you can help provide more information on the safety of this medicine.
Keep this medicine out of the sight and reach of children.
Store below 30°C. Store in the original package (carton) in order to protect from light and moisture.
Do not use the drug after the expiry date which is printed on the blister and carton after the abbreviation EXP Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer needed. These measures will help to protect the environment.
©Contents of the pack and other information
What Escitalopram contains
The active substance is escitalopram. Each Escitalopram Film-coated Tablet contains 5 mg, 10 mg, 15 mg or 20 mg escitalopram (as oxalate).
• The other ingredients are:
Core: microcrystalline cellulose, colloidal silica anhydrous, croscarmellose sodium, stearic acid and magnesium stearate. Coating: hypromellose (E464), macrogol 400, and titanium dioxide (E171).
What Escitalopram looks like and contents of the pack
Escitalopram 5 mg is a white, film-coated tablet, marked "93" on one side and "7414" on the other.
Escitalopram 10 mg is a white, film-coated tablet, scored on one side and marked "9" on one side and "3" on the other. The other side of the tablet is marked "7462" The tablet can be divided into equal halves.
Escitalopram 15 mg is a white, film-coated tablet, scored on one side and marked "S" on one side and "C" on the other. The other side of the tablet is marked "15"
The score line is only to facilitate breaking for ease of swallowing and not to divide into equal doses.
Escitalopram 20 mg is a white, film-coated tablet, scored on one side and marked "9" on one side and "3" on the other. The other side of the tablet is marked "7463"
The tablet can be divided into equal halves.
Escitalopram comes in blister packs of 7, 10, 14, 20, 28, 30, 49, 50, 56, 60, 90, 98, 100, 112, 120, 200 and 500 film-coated tablets and perforated unit dose blister 49x1, 50x1,
100x1 and 500x1 film-coated tablets. PVC/PVdC-Aluminium blisters are in the carton.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Teva UK Limited, Eastbourne, BN22 9AG,
UK.
This leaflet was last revised in 02/2014
PL 00289/1724 PL 00289/1725 PL 00289/1726 PL 00289/1727
PACKAGE LEAFLET: INFORMATION FOR THE USER
ESCITALOPRAM 5 mg, 10 mg, 15 mg AND 20 mg FILM-COATED TABLETS
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Escitalopram is and what it is used for
2. What you need to know before you take Escitalopram
3. How to take Escitalopram
4. Possible side effects
5. How to store Escitalopram
6. Contents of the pack and other information
OWhat Escitalopram is and what it is used for
Escitalopram belongs to a group of antidepressants called selective serotonin reuptake inhibitors (SSRIs). These medicines act on the serotonin-system in the brain by increasing the serotonin level. Disturbances in the serotonin-system are considered an important factor in the development of depression and related diseases. Escitalopram Film-coated Tablets contain escitalopram and are used to treat depression (major depressive episodes) and anxiety disorders (such as panic disorder with or without agoraphobia, social anxiety disorder, generalised anxiety disorder and obsessive-compulsive disorder).
What you need to know before you take Escitalopram
Do not take Escitalopram
• if you are allergic (hypersensitive) to escitalopram or any of the other ingredients of Escitalopram Film-coated Tablets (see section 6 "Contents of the pack and other information")
• if you take other medicines that belong to a group called MAO inhibitors, including selegiline (used in the treatment of Parkinson's disease), moclobemide (used in the treatment of depression) and linezolid (an antibiotic)
• if you are born with or have had an episode of abnormal heart rhythm (seen at ECG; an examination to evaluate how the heart is functioning)
• if you take medicines for heart rhythm problems or that may affect the heart's rhythm (see section 2 "Other medicines and Escitalopram").
Warnings and precautions
Talk to your doctor or pharmacist before
taking Escitalopram:
• if you have epilepsy. Treatment with Escitalopram should be stopped if seizures occur for the first time or if there is an increase in the seizure frequency (see also section 4 "Possible side effects")
• if you suffer from impaired liver or kidney function. Your doctor may need to adjust your dosage
• if you have diabetes. Treatment with Escitalopram may alter glycaemic control. Insulin and/or oral hypoglycaemic dosage may need to be adjusted • if you have a decreased level of sodium in the blood

• if you have a tendency to easily develop bleedings or bruises
• if you are receiving electroconvulsive treatment
• if you have coronary heart disease
• if you suffer or have suffered from heart problems or have recently had a heart attack
• if you have a low resting heart-rate and/or you know that you may have salt depletion as a result of prolonged severe diarrhoea and vomiting (being sick) or usage of diuretics (water tablets)
• if you experience a fast or irregular heartbeat, fainting, collapse or dizziness on standing up, which may indicate abnormal functioning of the heart rate
• if you have glaucoma (increased eye pressure).
Please note
Some patients with manic-depressive illness may enter into a manic phase. This is characterised by unusual and rapidly changing ideas, inappropriate happiness and excessive physical activity. If you experience this, contact your doctor.
Symptoms such as restlessness or difficulty to sit or stand still can also occur during the first weeks of the treatment. Tell your doctor immediately if you experience these symptoms.
Thoughts of suicide and worsening of your depression or anxiety disorder If you are depressed and/or have anxiety disorders you can sometimes have thoughts of harming or killing yourself. These may be increased when first starting antidepressants, since these medicines all take time to work, usually about two weeks but sometimes longer.
You may be more likely to think like this:
• if you have previously had thoughts about killing or harming yourself
• if you are a young adult. Information from clinical trials has shown an increased risk of suicidal behaviour in adults aged less than 25 years with psychiatric conditions who were treated with an antidepressant.
If you have thoughts of harming or killing yourself at any time, contact your doctor or go to a hospital straight away.
You may find it helpful to tell a relative or close friend that you are depressed or have an anxiety disorder and ask them to read this leaflet. You might ask them to tell you if they think your depression or anxiety is getting worse, or if they are worried about changes in your behaviour.
Children and adolescents under 18 years of age
Escitalopram should normally not be used for children and adolescents under 18 years. Also, you should know that patients under 18 have an increased risk of side effects such as suicide attempts, suicidal thoughts and hostility (predominately aggression, oppositional behaviour and anger) when they take this class of medicines. Despite this, your doctor may prescribe Escitalopram for patients under 18 because he/she decides that this is in their best interest. If your doctor has prescribed Escitalopram for a patient under 18 and you want to discuss this, please go back to your doctor. You should inform your doctor if any symptoms listed above develop or worsen when patients under 18 are taking Escitalopram. Also, the long term safety effects concerning growth, maturation and cognitive and behavioural development of Escitalopram in this age group have not yet been demonstrated.
Other medicines and Escitalopram Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicine.
Peel HereTo Open
Top of page cut-off to middle of registration mark = 44 mm
|
m |
Ref: 231-30-91411-B LEA ESCITALOPRAM A/S TAB TUK |
04 February 2014 | |||
|
TEVA UK LIMITED |
| Version: 10 |
Trackwise Parent: N/A |
Child: |
N/A | |
|
PL Number(s), MA Holder & Packer: |
PL 00289/1724-7. TEVA UK Limited Licence (Responsible Regulatory Officer = Packed at TEVA UK Limited, Eastbourne. Packing line: UHLMANN 1030. |
New Products Team), | |||
F. P. Code: 231-10-04981
231-10-04983 231-10-04985
Dimensions:
PANTONE® GREEN C
(PANTONE® is a registered
Pharma Code: Third party code: Fonts
Base Font Size:
868 (101100101)
N/A
Univers
Times (roman numerals only) 9 Pt
L:
W:
Folds to
323 mm 320 mm 160 mm
BLACK
Please refer to the latest version of the full base material specification: 92653, 92655, 92651
IMPORTANT: Artwork, text and content must not be reset, remade, amended or altered. The only exceptions to this are: bleeds, chokes, spreads or other print related adjustments required for reproduction by the supplier. We must receive a copy of any 3rd Party Supplier’s Proof before approval to print will be granted.
FOLD
LINE.
PAGE 2: LEFT HAND INSIDE FACE AFTER FOLDING AND TACKING
PAGE 3: RIGHT HAND INSIDE FACE AFTER FOLDING AND TACKING
Tell your doctor if you are taking any of the following medicines:
• "non-selective monoamine oxidase inhibitors (MAOIs)" containing phenelzine, iproniazid, isocarboxazid, nialamide and tranylcypromine as active ingredients. If you have taken any of these medicines you will need to wait 14 days before you start taking Escitalopram. After stopping Escitalopram you must allow 7 days before taking any of these medicines
• "reversible, selective MAO-A inhibitors" containing moclobemide (used to treat depression)
• "irreversible MAO-B inhibitors" containing selegiline (used to treat Parkinson's disease). These increase the risk of side effects
• the antibiotic linezolid
• lithium (used in the treatment of manic-depressive disorder) and tryptophan
• imipramine and desipramine (both used to treat depression)
• sumatriptan and similar medicines (used to treat migraine) and tramadol (used against severe pain). These increase the risk of a rare but potentially serious side effect known as serotonin syndrome
• cimetidine, omeprazole, esomeprazole, lansoprazole (used to treat stomach ulcers), fluvoxamine (antidepressant) and ticlopidine (used to reduce the risk of stroke). These may cause increased blood levels of Escitalopram
• St. John's Wort (hypericum perforatum) - a herbal remedy used for depression
• acetylsalicylic acid and non-steroidal anti-inflammatory drugs (medicines used for pain relief or to thin the blood, so called anti-coagulants). These may increase bleeding-tendency
• warfarin, dipyridamole, acenocumarol and phenprocoumon (medicines used to thin the blood, so called anti-coagulants). Your doctor will probably check the coagulation time of your blood when starting and discontinuing Escitalopram in order to verify that your dose of anti-coagulant is still adequate
• mefloquin (used to treat malaria), bupropion (used to treat depression) and tramadol (used to treat severe pain) due to a possible risk of a lowered threshold for seizures
• neuroleptics/antipsychotics (medicines to treat schizophrenia, psychosis) due to a possible risk of a lowered threshold for seizures, and antidepressants
• flecainide, propafenone and metoprolol (used in cardio-vascular diseases) clomipramine and nortriptyline (antidepressants) and risperidone, thioridazine and haloperidol (antipsychotics). The dosage of Escitalopram may need to be adjusted
• any drug which can decrease levels of potassium (hypokalaemia) or magnesium (hypomagnesaemia) in blood.
Do not take Escitalopram if you take medicines for heart rhythm problems or medicines that may affect the heart's rhythm, such as Class IA and III antiarrhythmics, antipsychotics (e.g. phenothiazine derivatives, pimozide, haloperidol), tricyclic antidepressants, certain antimicrobial agents (e.g. sparfloxacin, moxifloxacin, erythromycin IV, pentamidine, anti-malarian treatment particularly halofantrine), certain antihistamines (astemizole, mizolastine). If you have any further questions about this you should speak to your doctor.
Escitalopram with food, drink and alcohol
Escitalopram can be taken with or without food (see section 3 "How to take Escitalopram").
As with many medicines, combining Escitalopram with alcohol is not advisable, although Escitalopram is not expected to interact with alcohol.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine. Do not take Escitalopram if you are pregnant or breast-feeding unless you and your doctor have discussed the risks and benefits involved.
Citalopram, a medicine like escitalopram, has been shown to reduce the quality of sperm in animal studies. Theoretically, this could affect fertility, but impact on human fertility has not been observed as yet.
If you take Escitalopram during the last 3 months of your pregnancy you should be aware that the following effects may be seen in your newborn baby: trouble with breathing, bluish skin, fits, body temperature changes, feeding difficulties, vomiting, low blood sugar, stiff or floppy muscles, vivid reflexes, tremor, jitteriness, irritability, lethargy, constant crying, sleepiness and sleeping difficulties. If your newborn baby has any of these symptoms, please contact your doctor immediately.
Make sure your midwife and/or doctor know you are on Escitalopram. When taken during pregnancy, particularly in the last 3 months of pregnancy, medicines like Escitalopram may increase the risk of a serious condition in babies, called persistent pulmonary hypertension of the newborn (PPHN), making the baby breathe faster and appear bluish. These symptoms usually begin during the first 24 hours after the baby is born. If this happens to your baby you should contact your midwife and/or doctor immediately.
If used during pregnancy Escitalopram should never be stopped abruptly.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
You are advised not to drive a car or operate machinery until you know how Escitalopram affects you.
How to take Escitalopram
Always take Escitalopram exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
Adults
Depression
The normally recommended dose of Escitalopram is 10 mg taken as one daily dose. The dose may be increased by your doctor to a maximum of 20 mg per day.
Panic disorder
The starting dose of Escitalopram is 5 mg as one daily dose for the first week before increasing the dose to 10 mg per day. The dose may be further increased by your doctor to a maximum of 20 mg per day.
Social anxiety disorder
The normally recommended dose of Escitalopram is 10 mg taken as one daily dose. Your doctor can either decrease your dose to 5 mg per day or increase the dose to a maximum of 20 mg per day, depending on how you respond to the medicine. Generalised anxiety disorder
The normally recommended dose of Escitalopram is 10 mg taken as one daily dose. The dose may be increased by your doctor to a maximum of 20 mg per day.
Obsessive-compulsive disorder
The normally recommended dose of Escitalopram is 10 mg taken as one daily dose. The dose may be increased by your doctor to a maximum of 20 mg per day.
Older people (above 65 years of age)
The recommended starting dose of Escitalopram is 5 mg taken as one daily dose. The dose may be increased by your doctor to 10 mg per day.
Use in children and adolescents (below 18 years of age)
Escitalopram should not normally be given to children and adolescents. For further information please see section 2 "What you need to know before you take Escitalopram"
You can take Escitalopram with or without food. Swallow the tablet with some water. Do not chew them, as the taste is bitter.
Escitalopram 10 mg Film-coated Tablets, Escitalopram 20 mg Film-coated Tablets
If necessary, you can divide the tablets by firstly placing the tablet on a flat surface with the score facing upwards. The tablets may then be broken by pressing down on each end of the tablet, using both forefingers as shown in the drawing.
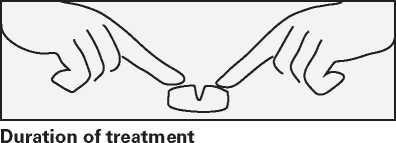
It may take a couple of weeks before you start to feel better. Continue to take Escitalopram even if it takes some time before you feel any improvement in your condition.
Do not change the dose of your medicine without talking to your doctor first.
Continue to take Escitalopram for as long as your doctor recommends. If you stop your treatment too soon, your symptoms may return. It is recommended that treatment should be continued for at least 6 months after you feel well again.
If you take more Escitalopram than you should
If you take more than the prescribed dose of Escitalopram, contact your doctor or nearest hospital emergency department immediately. Do this even if there are no signs of discomfort. Some of the signs of an overdose could be dizziness, tremor, agitation, convulsion, coma, nausea, vomiting, change in heart rhythm, decreased blood pressure and change in body fluid/salt balance. Take the Escitalopram box/container with you when you go to the doctor or hospital .
If you forget to take Escitalopram
Do not take a double dose to make up for forgotten doses. If you do forget to take a dose and you remember before you go to bed, take it straight away. Carry on as usual the next day. If you only remember during the night, or the next day, leave out the missed dose and carry on as usual.
If you stop taking Escitalopram Do not stop taking Escitalopram until your doctor tells you to do so. When you have completed your course of treatment, it is generally advised that the dose of Escitalopram is gradually reduced over a number of weeks.
When you stop taking Escitalopram, especially if it is abruptly, you may feel discontinuation symptoms. These are common when treatment with Escitalopram is stopped. The risk is higher, when Escitalopram has been used for a long time or in high doses or when the dose is reduced too quickly. Most people find that the symptoms are mild and go away on their own within two weeks. However, in some patients they may be severe in intensity or they may be prolonged (2-3 months or more). If you get severe discontinuation symptoms when you stop taking Escitalopram, please contact your doctor. He or she may ask you to start taking your tablets again and come off them more slowly.
Discontinuation symptoms include: Feeling dizzy (unsteady or off-balance), feelings like pins and needles, burning sensations and (less commonly) electric shock sensations, including in the head, sleep disturbances (vivid dreams, nightmares, inability to sleep), feeling anxious, headaches, feeling sick (nausea), sweating (including night sweats), feeling restless or agitated, tremor (shakiness), feeling confused or disorientated, feeling emotional or irritable, diarrhoea (loose stools), visual disturbances, fluttering or pounding heartbeat (palpitations).
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Possible side effects
Like all medicines, Escitalopram can cause side effects, although not everybody gets them.
The side effects usually disappear after a few weeks of treatment. Please be aware that many of the effects may also be symptoms of your illness and therefore will improve when you start to get better.
If you experience the following side effects you should contact your doctor or go to the hospital straight away:
Uncommon (may affect up to 1 in 100 people):
• unusual bleeds, including gastrointestinal bleeds.
Rare (may affect up to 1 in 1,000 people):
• if you experience swelling of skin, tongue, lips, or face, or have difficulties breathing or swallowing (allergic reaction), contact your doctor or go to a hospital straight away
• if you have a headache, increased heart rate, shivering, high fever, sweating, agitation, confusion, nausea or diarrhoea, trembling and abrupt contractions of muscles these may be signs of a rare but potentially life-threatening condition called serotonin syndrome. If you feel like this contact your doctor.
Some patients have reported (frequency can not be estimated from the available data)
• fast, irregular heart beat, fainting which could be symptoms of a life-threatening condition known as Torsades de Pointes
• difficulties urinating
• seizures (fits), see also section "Warnings and precautions"
• yellowing of the skin and the white in the eyes are signs of liver function impairment/ hepatitis.
In addition to above the following side effects have been reported:
Very common (may affect more than 1 in 10 people):
• feeling sick (nausea)
• headache.
Common (may affect up to 1 in 10 people):
• blocked or runny nose (sinusitis)
• decreased or increased appetite
• anxiety, restlessness, abnormal dreams, difficulties falling asleep, feeling sleepy, dizziness, yawning, tremors, prickling of the skin
• diarrhoea, constipation, vomiting, dry mouth
• increased sweating
• pain in muscle and joints (arthralgia and myalgia)
• sexual disturbances (delayed ejaculation, problems with erection, decreased sexual drive and women may experience difficulties achieving orgasm)
• fatigue, fever
• increased weight.