Espranor 8 Mg Oral Lyophilisate
PACKAGE LEAFLET: INFORMATION FOR THE USER
D02813
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See Section 4.
(Buprenorphine)
Espranor with food, drink and alcohol
Espranor should not be taken at the same time as food or drink.
You should not drink alcohol or take any medicines
What is in this leaflet:
1. What Espranor is and what it is used for
2. What you need to know before you take Espranor
3. How to take Espranor
4. Possible side effects
5. How to store Espranor
6. Contents of the pack and other information
1. What Espranor Oral Lyophilisate is and what it is used for
Espranor oral lyophilisate is a freeze-dried wafer which dissolves rapidly on the tongue.
Espranor is used in adults and adolescent over 15 years of age, as part of a medical, social and psychological treatment programme for addiction.
Espranor contains buprenorphine, an opioid (narcotic) analgesic. When it is used for the treatment of patients addicted to opiate (narcotic) drugs, such as morphine or heroin, it acts as a substitute for these drugs and therefore aids the patient in withdrawing from them over a period of time.
If treatment is stopped abruptly, withdrawal symptoms can occur.
2. What you need to know before you take Espranor
that contain alcohol while taking Espranor as this will ncrease the risk of drowsiness, respiratory depression and fatal overdose.
Pregnancy, breast-feeding and fertility
3efore taking Espranor, tell your doctor if you are pregnant or trying to become pregnant. If you become pregnant during treatment with Espranor, tell your doctor straight away.
Since Espranor is passed into breast milk, you should not breast feed while taking this medicine.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
This medicine can cause drowsiness, which may be made worse if you also drink alcohol or take tranquilizers or anti-anxiety drugs. If you are drowsy, do not drive or operate machinery.
This medicine can affect your ability to drive.
Do not drive whilst taking this medicine until you know how this medicine affects you. t may be an offence to drive if your ability to drive safely is affected.
There is further information for patients who are ntending to drive in Great Britain - go to https://
Talk to your doctor or pharmacist if you are not sure whether it is safe for you to drive while taking this medicine
Important information about some of the ingredients of Espranor
Espranor contains aspartame. Aspartame contains a source of phenylalanine. This may be harmful for people with phenylketonuria.
3. How to take Espranor
Always take Espranor exactly as your doctor or pharmacist has told you. You should check with your doctor or pharmacist if you are not sure.
When to start taking Espranor Starting Espranor treatment if you are dependent on heroin or a short acting opioid - your first dose of Espranor should be taken at least 6 hours after you ast used the opioid or when signs of withdrawal appear.
Starting Espranor treatment if you are dependent on methadone or a long acting opioid - you will not start treatment with this medicine until your daily dose of methadone is 30 mg a day or less. The first dose of Espranor should be taken when signs of withdrawal appear, but not less than 24 hours after you last used methadone.
:or the first 24 hours of treatment, you may feel uncomfortable with some mild opiate withdrawal symptoms e.g. sweating, feeling sick (see section 4 Possible side effects).
How much to take
Your doctor will decide what dose you need to start treatment with.
During treatment your doctor will adjust your dose depending upon your response. The maximum dose s 18 mg daily. After a period of successful treatment, your doctor may gradually reduce your dose and depending on your condition, may stop it altogether.
Do not suddenly stop taking Espranor as this may lead to withdrawal symptoms.
Instructions for use
Rare side effects (may effect up to 1 in 1000 people) are:
Slow or difficult breathing, liver injury with or without jaundice, swelling of face and throat or life threatening allergic reactions.
Side effects with unknown frequency (frequency cannot be estimated from the available data) include: Sudden withdrawal syndrome caused by taking
Espranor is not interchangeable with other oral buprenorphine products and the dose of Espranor may differ from the dose of other buprenorphine products
Do not take Espranor if:
You have severe breathing problems
You have severe liver problems
You are alcohol dependent or suffer from acute
alcoholism including 'the shakes' or hallucinations
You are pregnant (unless your doctor tells you to
take it)
You are allergic (hypersensitive) to buprenorphine or any of the other ingredients in Espranor (see section 6)
Warnings and Precautions
Talk to your doctor, pharmacist or nurse before taking Espranor:
If you suffer breathing problems e.g. asthma If you have kidney problems If you have liver problems If you are breastfeeding a baby
If any of the above applies to you, please tell your doctor before taking Espranor as your doctor may need to reduce your dose of Espranor or you may need additional treatment to control it.
Espranor should not be given to children or adolescents under 15 years old.
Other medicines and Espranor
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
The following medicines have sedative effects (make you feel sleepy/drowsy). These effects are increased if these medicines are taken while you are being treated with Espranor:
Benzodiazepines (medicines used to treat anxiety
or sleep disorders) e.g. diazepam (valium). Your doctor will prescribe the correct dose for you. Taking the wrong dose of benzodiazepines may cause death due to respiratory failure (inability to breathe).
• Barbiturates e.g. phenobarbital
• Other opioids or opioid derivatives e.g. morphine, strong pain killers or cough medicines
• Certain antidepressants e.g. fluoxetine (Prozac)
• Monoamine oxidase inhibitors (MAOI) (medicines used to treat severe depression) e.g. phenelzine
• Medicines that cause drowsiness such as antihistamines or sedatives
• Certain drugs used to treat high blood pressure
• Antipsychotic drugs (medicines used to treat certain mental disorders)
If you are taking any of the following medicines, your doctor may need to prescribe a lower dose of Espranor:
Ketoconazole (medicine used to treat a fungal infection which can increase the levels of Espranor in your blood if both are taken at the same time) Gestodene (found in some contraceptive pills) Drugs used to treat HIV e.g. ritonavir, indinavir and saquinavir
Phenprocoumon (a blood thinning medicine)
If you are taking any of the following medicines, your doctor may need to prescribe a higher dose of Espranor:
Enzyme inducers e.g. phenobarbital, carbamazepine, phenytoin and rifampicin
Naltrexone may prevent the therapeutic effects of Espranor. If currently taking this medicine followed by concomitant use of naltrexone, you may experience a sudden onset of prolonged and intense withdrawal.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
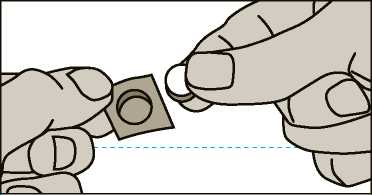
Espranor is sensitive to moisture. Make sure your hands are dry before handling the wafer. Take the wafer by following the instructions below:
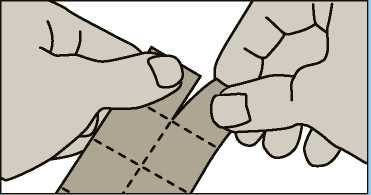
. Tear a square off the blister pack along the perforated lines.
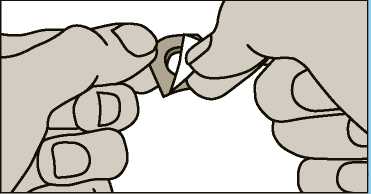
2. The foil is easily peelable. Do not force the wafer through the foil as it is fragile and can easily break. nstead, fold back the foil and then peel it off.
Continued overleaf
Take Espranor by placing ON your tongue, not
under your tongue.
3. Remove the wafer carefully from the foil and take out from the packaging immediately.
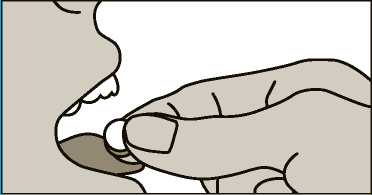
4. Place the wafer on the tongue and close your mouth. Allow it to remain there for a few seconds until it has dissolved. Try to avoid swallowing during he first 2 minutes.
Do not eat or drink for at least 5 minutes. f you take more Espranor than you should
"ell your doctor immediately or contact your nearest hospital casualty department. Remember to take the
pack and any remaining wafers with you. f you forget to take Espranor
You should tell your doctor and follow their nstructions. Do not take a double dose to make up for he missed dose, unless your doctor tells you to. f you stop taking Espranor Do not suddenly stop taking the wafers unless told :o do so by your doctor, as this may cause withdrawal ymptoms.
f you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects .ike all medicines, this medicine can cause side ffects, although not everyone gets them.
or the first 24 hours of treatment, you may feel uncomfortable with some mild opiate withdrawal ymptoms.
Tell your doctor immediately or seek urgent medical attention if you experience uncommon side ffects, such as:
• swelling of the face, lips, tongue or throat which may cause difficulty in swallowing or breathing, severe hives/nettle rash. These may be signs of a life-threatening allergic reaction.
• feeling sleepy and uncoordinated, have blurred vision, have slurred speech, cannot think well or clearly, or your breathing gets much slower than is normal for you.
Also tell your doctor immediately if you experience uncommon side effects such as: severe tiredness, itching with yellowing of skin or eyes. These may be symptoms of liver damage. seeing of hearing things that are not there (hallucination)
Very common side effects (may effect more than 1 in 10 people) include:
nsomnia (inability to sleep), constipation, feeling or being sick (nausea), sweating, headache, drug withdrawal syndrome.
ommon side effects (may effect up to 1 in 10 people) are:
Weight loss, swelling (hands and feet), tiredness, drowsiness, anxiety, nervousness, tingling, depression, decreased sexual drive, increase in muscle tension, abnormal thinking, increased earing (watering eyes) or other tearing disorder, blurred vision, flushing, increased blood pressure, palpitations, widening of blood vessel, migraines, unny nose, sore throat and painful swallowing, ncreased cough, upset stomach or other stomach discomfort, diarrhoea, abnormal liver function, latulence, vomiting, numbness of the tongue or mouth, rash, itching, hives, pain, joint pain, muscle pain, leg cramps (muscle spasm), difficulty in getting or keeping an erection, urine abnormality, abdominal pain, back pain, weakness, infection, hills, chest pain, fever, flu-like symptoms, feeling of general discomfort, accidental injury caused by loss of alertness or co-ordination, faintness and dizziness, drop in blood pressure on changing position from itting or lying down to standing.
Uncommon side effects (may effect up to 1 in 100 people) are:
Swollen glands, agitation, tremor, abnormal dream, excessive muscle activity. Not feeling like yourself , medicine dependence, amnesia (memory disturbance), loss of interest, exaggerated feeling of well-being, convulsion (fits), speech disorder, small pupil size, difficulty urinating, eye inflammation or infection, rapid or slow heart beat, low blood pressure, myocardial infarction (heart attack), chest ightness, Shortness of breath, asthma, yawning, pain and sores in mouth, tongue discolouration, acne, skin nodule, hair loss, dry or scaling skin, nflammation of joints, urinary tract infection, abnormal blood tests, loss of appetite, blood in urine, abnormal ejaculation, menstrual or vaginal problems, kidney stone, sensitivity to heat or cold, ieat stroke, feelings of hostility.
Espranor too soon after use of illicit opioids, drug withdrawal syndrome in newborn.
f any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
f you are not sure what the side effects listed are, ask your doctor to explain them to you.
Reporting of side effects
f you get any side effects, talk to your doctor or pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report ide effects directly via the Yellow Card Scheme Website: http//www.mhra.gov.uk/yellowcard.
By reporting the side effects you can help provide more information on the safety of this medicine.
5. How to store Espranor
Keep this medicine out of the sight and reach of children
Do not use Espranor after the expiry date, which is tated on the carton after EXP. The expiry date refers to the last day of that month.
Store your medicine in the original packaging to protect from light and moisture.
Espranor does not require any special temperature torage conditions.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help to protect the environment.
6. Contents of the pack and other information
What Espranor contains:
The active substance is buprenorphine (as buprenorphine hydrochloride). Each wafer contains 2 mg and 8 mg of buprenorphine.
■ The other ingredients are gelatin, mannitol, aspartame, mint flavour and citric acid.
What Espranor looks like and the contents of the pack
Espranor 2 mg oral lyophilisate is a white to off-white circular wafer marked with "M2” on one side.
Espranor 8 mg oral lyophilisate is a white to off-white circular wafer marked with "M8” on one side.
Your medicine is available in blisters containing 7 or
28 wafers in an outer carton.
Marketing Authorisation Holder and Manufacturer:
Martindale Pharmaceuticals Ltd Trading as Martindale Pharma)
Bampton Road Harold Hill Romford, Essex RM38UG United Kingdom.
This leaflet was last updated in May 2015 Reference Number:
Espranor 2 mg Oral Lyophilisate: PL 00156/0364 Espranor 8 mg Oral Lyophilisate: PL 00156/0365
M
D02813
2813-J