Estraderm Mx 25
Out of date information, search anotherSUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Estraderm MX 25®
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
The active ingredient is estra-1, 3,5(10)-triene-3,17B-diol (oestradiol hemihydrate).
Patches contain 0.75 mg active substance corresponding to a surface area of 11cm2.
For a full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Estraderm MX is a square-shaped, self-adhesive, transparent, transdermal patch for application to the skin surface. Each patch comprises an impermeable polyester backing film, an adhesive matrix containing estradiol and an oversized protective liner which is removed prior to application of the patch to the skin. Estraderm MX releases estradiol into the circulation via intact skin at a low rate for up to 4 days.
Cross section:
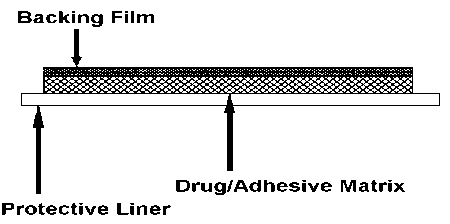
|
DOSAGE STRENGTHS |
ESTRADERM MX 25 |
|
Nominal rate of estradiol release |
25 micrograms /day |
|
Estradiol content |
0.75mg |
|
Drug-releasing area |
11 cm2 |
|
Imprint |
Product logo |
|
(on backing film) |
CG GRG |
4. CLINICAL PARTICULARS
4.1. Therapeutic Indications
Hormone replacement therapy (HRT) for oestrogen deficiency symptoms in postmenopausal women.
The experience treating women older than 65 years is limited.
4.2 Posology and method of administration
Estraderm MX 25 is an oestrogen only patch.
In women with an intact uterus oestrogen should be supplemented by sequential administration of a progestagen (e.g. medroxyprogesterone acetate 10mg, norethisterone 5mg, norethisterone acetate 1-5mg or dydrogesterone 20mg per day) to be taken at least on the last 12 days of each 4-week treatment cycle. Withdrawal bleeding usually occurs following 12 days or more of progesterone administration. Unless there is a previous diagnosis of endometriosis, it is not recommended to add a progestagen in hysterectomised women.
Dosage
Adults and Elderly
Menopausal symptoms: For initiation and continuation of treatment of postmenopausal symptoms, the lowest effective dose for the shortest duration should be used (see also section 4.4). Depending on the clinical response the dose can then be adjusted to the patient’s individual needs. If, after three months, there is insufficient response in the form of alleviated symptoms, the dose can be increased.
A maximum dose of 100 micrograms per day should not be exceeded.
Effects usually of estrogenic origin e.g. breast discomfort, water retention or bloating are often observed at the start of treatment, especially in patients receiving hormone replacement therapy for the first time. However, if symptoms persist for more than six weeks the dose should be reduced.
General instructions: Estraderm MX is administered as a continuous treatment (uninterrupted application twice weekly).
For most postmenopausal women not taking HRT Estraderm MX therapy may be started at any convenient time. However, for women with an intact uterus who are still menstruating regularly, commencement within 5 days of the onset of bleeding is recommended.
In women with an intact uterus transferring from a continuous sequential HRT regimen, treatment should begin the day following completion of the prior regimen.
In women transferring from a continuous-combined HRT regimen, or hysterectomised women transferring from other oestrogen-only HRT treatment, treatment may be started on any convenient day.
Administration: Estraderm MX should be applied immediately after removal of the protective liner (see Figs.), to an area of clean, dry, and intact skin on the trunk below the waistline. The site chosen should be one at which little wrinkling of skin occurs during movement of the body, e.g. buttock. Estraderm MX should never be applied to, or near the breasts.
Estraderm MX should be applied twice weekly on a continuous basis, each used patch being removed after 3-4 days and a fresh system applied to a slightly different site.
If a woman has forgotten to apply a patch, she should apply a new patch as soon as possible. The subsequent patch should be applied according to the original treatment schedule. The interruption of treatment might increase the likelihood of recurrence of symptoms and include breakthrough spotting and bleeding.
In the event that a patch should fall off a new patch may be applied. The original treatment schedule should be continued.
The patch should not be exposed to sunlight.
Special populations
Patients with renal and /or hepatic impairment
No studies were performed in patients with renal and hepatic impairment.
All oestrogen preparations are contraindicated in patients with severe hepatic impairment (see section 4.3 contra-indications).
Children
Estraderm MX is not indicated for use in children.
4.3 Contra-indications
Estraderm MX should not be used by women with any of the following
conditions:
• Known, past or suspected breast cancer
• Known or suspected oestrogen-dependent malignant tumours (e.g. endometrial cancer)
• Undiagnosed genital bleeding
• Untreated endometrial hyperplasia
• Previous or current venous thromboembolism (deep venous thrombosis, pulmonary embolism)
• Known thrombophilic disorders (e.g. protein C, protein S, or antithrombin deficiency, see section 4.4)
• Active or recent arterial thromboembolic disease (e.g. angina, myocardial infarction)
• Acute liver disease, or a history of liver disease as long as liver function tests have failed to return to normal
• Known hypersensitivity to the active substance or to any of the excipients
• Porphyria
4.4 Special warnings and precautions for use
For the treatment of postmenopausal symptoms, HRT should only be initiated for symptoms that adversely affect quality of life. In all cases, a careful appraisal of the risks and benefits should be undertaken at least annually and HRT should only be continued as long as the benefit outweighs the risk.
Evidence, .regarding, the. .risks _ associated, with hrt in. the. treatment, of premature, .m.e.n.op.aus.e . is .l.i.m.i.t.ed;. .Due . to .the. . low. . .Level . of .absolute. . risk . In. .younger. .w.o.m.en, . .h.o.we.v.e.r, .the. . balance . of benefits, and .ri.s.ks . for these. wo.m_e.n_ _m_ay .be. more.favourable .than. in . older .w.o.m.en.
Medical Examination / follow-up
Before initiating or reinstituting HRT, a complete personal and family medical history should be taken. Physical (including pelvic and breast) examination should be guided by this and by the contraindications and warnings for use. During treatment, periodic check-ups are recommended of a frequency and nature adapted to the individual woman. Women should be advised what changes in their breasts should be reported to their doctor or nurse (see ‘Breast cancer’ below). Investigations, including appropriate imaging tools e.g. mammography, should be carried out in accordance with currently accepted screening practices, modified to the clinical needs of the individual.
Conditions which need supervision
If any of the following conditions are present, have occurred previously, and/or have been aggravated during pregnancy or previous hormone treatment, the patient should be closely supervised. It should be taken into account that these conditions may recur or be aggravated during treatment with Estraderm MX, in particular:
• Leiomyoma (uterine fibroids) or endometriosis
• Risk factors for thromboembolic disorders (see below)
• Risk factors for oestrogen dependent tumours, e.g. 1st degree heredity for breast cancer
• Hypertension
• Liver disorders (e.g. liver adenoma)
• Diabetes mellitus with or without vascular involvement
• Cholelithiasis
• Migraine or (severe) headache
• Systemic lupus erythematosus
• A history of endometrial hyperplasia (see below)
• Epilepsy
• Asthma
• Otosclerosis
Reasons for immediate withdrawal of therapy
Therapy should be discontinued in case a contra-indication is discovered and in the following situations:
• Jaundice or deterioration in liver function
• Significant increase in blood pressure
• New onset of migraine-type headache • Pregnancy
Endometrial hyperplasia and carcinoma
In women with an intact uterus the risk of endometrial hyperplasia and carcinoma is increased when oestrogens are administered alone for prolonged periods. The. .reported . increase . in endometrial .cancer._ risk. . among .. P.esfro.ge.n.:Qnly_. users. ..varie.s. . from. . 2-to .. 12_-_fold ..greater compared..with. . non-users,. depending.. on . the. .duration. of. treatment . and . P_e.stro.gen. .dose. .(see section. 4.8).. After. stopping. treatment .risk. may .re.m.ain. elevated . for at least. iP. .years.
The addition of a progestagen cyclically for at least 12 days per month/28 day cycle or continuous combined oestrogen-progestagen therapy in non-hysterectomised women prevents the excess risk associated with oestrogen-only HRT.. Withdrawal bleeding usually occurs following the 12 days or more of progestagen administration.
Break-through bleeding and spotting may occur during the first months of treatment. If break-through bleeding or spotting appears after some time on therapy, or continues after treatment has been discontinued, the reason should be investigated, which may include endometrial biopsy to exclude endometrial malignancy.
Unopposed oestrogen stimulation may lead to premalignant or malignant transformation in the residual foci of endometriosis. Therefore, the addition of progestagens to oestrogen replacement therapy should be considered in women who have undergone hysterectomy because of endometriosis, if they are known to have residual endometriosis.
Breast cancer
The. overall evidence. suggests an .increased. risk pfbre.ast. cancer .in .women .taking. combined oestrogen. -. prog.es.t.age.n. .and. possibly .also . oestrogen-only .HRT, that is dependent. on .the duration . oftaking .HRT.
Combined. oestrogen-progestagen .therapy
The randomised placebo-controlled trial, the Women’s Health Initiative study (WHI), and
epidemiological studies are consistent in finding an increased risk of breast cancer in women taking combined oestrogen-progestagen for HRT that becomes apparent after about 3 years (see section 4.8).
Oestrogen-only. therapy.
The . WHI . trial. found. no. increa.se . in . the. . risk . of breast .cancer. .in .hysterectomised . wo.m.e.n. .using .oestrogen-only. . HRT.-. . Observational. .studies . .have . .mostly. . reported. . a . small. . increase, in . risk . .of having breast. cancer. diagnosed. that. is .substantially. lower.than that. found . in . users, .of. o.e.strogen-progestagen. cp.mbinatio.ns .(see .section. 4.8.).
The excess risk becomes apparent within a few years of use but returns to baseline within a few (at most five) years after stopping treatment.
HRT, especially oestrogen-progestagen combined treatment, increases the density of mammographic images which may adversely affect the radiological detection of breast cancer.
Venous thromboembolism
HRT is associated with a 1..3-3. fold risk of developing venous thromboembolism (VTE), i.e. deep vein thrombosis or pulmonary embolism.
The occurrence of such an event is more likely in the first year of HRT than later (see section 4.8).
Generally recognised risk factors for VTE include use of oestrogens, older age, major surgery, prolonged immobilisation, obesity (Body Mass Index > 30kg/m2), pregnancy/postpartum period, systemic lupus erythematosus (SLE) and cancer. There is no consensus about the role of varicose veins in VTE.
Patients with known thrombophilic states have an increased risk of VTE. and HRT may add to this risk. HRT is therefore contraindicated in these patients (see section 4.3) Women already on chronic anticoagulant treatment require careful consideration of the benefit-risk of use of HRT.
As in all post-operative patients prophylactic measures need to be considered to prevent VTE following surgery. If prolonged immobilisation is to follow elective surgery, temporarily stopping HRT four to six weeks earlier is recommended. Treatment should not be restarted until the woman is completely mobilised.
In. .women . with _ no. p.ers.o.n.al. .history, .o.f. V.TE. but with _ a. _ first. .d_e_gre.e. .relative, .with. _a_ .history, of thrombosis, .at . young . .age., . screening . may. be. . offered . .after . .ca.re.fu! . .counselling. .regarding. . its limitations. . .(o.n.ly. . .a. .proportion . .o.f. thrombo.p.hilic. . defects. . are. . identified. .by . .screening).. . .If. .a thrombophilic . de.fe.ct .is. identified. which . segregates . with .thrombosis. in. .family, .members .or. if the . de.fe.ct. is . .‘sey.er.ei (e.g,. antithrombin, . protein . S, or protein. C . deficie.n.cie.s. or .a. .combination of defects). HRT. is .contraindicated.
If VTE develops after initiating therapy, the drug should be discontinued. Patients should be told to contact their doctors immediately when they are aware of a potential thromboembolic symptom (e.g. painful swelling of a leg, sudden pain in the chest, dyspnoea).
Coronary artery disease (CAD)
HRT should not be used to prevent cardiovascular disease.
There is no evidence from randomised controlled trials of protection ..against .myocardial infarction . .in . . women. _ with. _ or . .without. _ exi sting. . C AD. . .who. _ received. _ combined. _ oe stroge.n.-. progestagen .or oestroge.n-on.iy. .H.RT..
.Combined. oe.strogen-progestage.n. .therapy
The. relative .risk, of CAD. during use of combined oestrogen and .progestagen. HRT _is slightly increased.. As the. baseline . absolute, .risk. of CAD is . strongly, dep.endent on ,age=.the. .number of extra .cases .of. CAD. due. .to. .oestrogen. and. progestagen. use. is .very. low. in .healthy. women. close to. menopause, but. will . rise. with. more . advanced, age..
Oestrogen-only
Randomised, controlled, .data. found .no .increased. risk .of. CAD . in hysterectomised women .using oes.trogen.-on.ly. therapy-
Ischaemic stroke
.Combined, .oestrogen-progestagen. . and . _oestr.ogen-.only. therapy, .are. . associated . with. .an . up . .to _1,5-_fo_ld_ .increase, .in . risk . of .ischaemic. .stroke. . The. relative. risk. .does . not . change. .with. .age. or time. since . menopause. However, .as .the .baseline. risk. of .stroke . is .strongly, age-dependent, .the overall, .risk .o.f.stroke. in women .w.h_o .use .HRT will. increase .with. age. .(see .section. 4,8)..
Ovarian cancer
Ovarian cancer is much rarer that breast cancer. Long-term (at least 5 to 10 years) use of oestrogen-only HRT products has been associated with a slightly increased risk of ovarian cancer (see section 4.8)
Some . .studies, .including, .the. whi . trial. suggest .that .the. .long-term .use. of .combined. .HRTs . may. confer. a .similar, or. slightly .smaller, .risk (see .section. 4,8).
Severe anaphylactic/anaphylactoid reactions and angioedema
Cases of anaphylactic/anaphylactoid reactions, which developed anytime during the course of Estraderm treatment and required emergency medical management, have been reported in the post marketing setting. Involvement of skin (uticaria, pruritus, swelling of the face, throat, lips, tongue, skin and periorbital edema) and either respiratory tract (respiratory compromise) or gastrointestinal tract (abdominal pain, vomiting) has been noted. Angioedema requiring medical intervention involving the eye/eyelid, face, larynx, pharynx, tongue and extremities (hands, legs, ankles and fingers) with or without urticaria has occurred in the post marketing experience of using Estraderm. If angioedema involves the tongue, glottis, or larynx, airway obstruction may occur. Patients who develop angioedema after treatment with Estraderm should not receive Estraderm again.
Oestrogens. .may. induce, or exacerbate . symptoms, .of . angioedema, in. .particular in women .with hereditary angioedem.a.
Other conditions
Oestrogens may cause fluid retention, and therefore patients with cardiac or renal dysfunction should be carefully observed.
Women with pre-existing hypertriglyceridemia should be monitored closely during oestrogen replacement or hormone replacement therapy, since rare cases of large increases in plasma triglycerides leading to pancreatitis have been reported with oestrogen therapy in this condition.
Oestrogens increase thyroid binding globulin (TBG), leading to increased circulating total thyroid hormone, as measured by protein-bound iodine (PBI), T4 levels (by column or by radio-immunoassay) or T3 levels (by radio-immunoassay). T3 resin uptake is decreased, reflecting the elevated TBG. Free T4 and free T3 concentrations are unaltered. Other binding proteins may be elevated in serum, i.e. corticoid binding globulin (CBG), sex-hormonebinding globulin (SHBG) leading to increased circulating corticosteroids and sex steroids, respectively. Free or biological active hormone concentrations are unchanged. Other plasma proteins may be increased (angiotensinogen/renin substrate, alpha-I-antitrypsin, ceruloplasmin). These effects may be less common with transdermal oestradiol than with oral oestrogens.
Contact sensitisation is known to occur with all topical applications. Although it is extremely rare, patients who develop contact sensitisation to any of the components of the patch should be warned that a severe hypersensitivity reaction may occur with continuous exposure to the causative agent.
Although observations to date suggest that oestrogens, including transdermal oestradiol, do not impair carbohydrate metabolism, diabetic women should be monitored during initiation of therapy until further information is available.
Thyroid function should be monitored regularly in patients who require thyroid hormone replacement therapy and who are also taking oestrogen in order to ensure that thyroid hormone levels remain within an acceptable range.
Women should be advised that Estraderm MX is not a contraceptive, nor will it restore fertility. Women requiring contraception should be advised to use non-hormonal contraception.
HRT use does not improve cognitive function. There is some evidence of increased risk of probable dementia in women who start using continuous combined or oestrogen-only HRT after the age of 65.
4.5 Interaction with other medicinal products and other forms of interaction
The metabolism of oestrogens may be increased by concomitant use of substances known to induce drug-metabolising enzymes, specifically cytochrome P450 enzymes, such as anticonvulsants (e.g. phenobarbital, phenytoin, carbamazepine) and anti-infectives (e.g. rifampicin, rifabutin, nevirapine, efavirenz).
Oestradiol is predominantly metabolized by CYP3A4; concomitant administration of inhibitors of CYP3A4 such as ketoconazole, erythromycin or ritonavir may therefore result in an increase of approximately 50% in oestradiol exposure.
Caution should be used if the women is receiving protease inhibitors (e.g.ritonavir and nelfinavir), which are known as strong inhibitors of cytochrome P450 enzymes, and by contrast exhibit inducing properties when used concomitantly with steroid hormones.
Herbal preparations containing St John’s wort (Hypericum perforatum) may induce the metabolism of oestrogens and progestagens.
With transdermal HRT administration, the first-pass effect in the liver is avoided and, thus transdermally applied oestrogens may be less affected by enzyme inducers than oral hormones.
Clinically, an increased metabolism of oestrogens and progestagens may lead to decreased effects and changes in the uterine bleeding profile.
Some laboratory tests may be influenced by oestrogen therapy, such as tests for glucose tolerance or thyroid function.
4.6 Pregnancy and lactation
Pregnancy
Estraderm MX is not indicated during pregnancy. If pregnancy occurs during medication with Estraderm MX treatment should be withdrawn immediately.
The results of most epidemiological studies to date relevant to inadvertant foetal exposure to oestrogens indicate no teratogenic or foetotoxic effects.
Lactation
Estraderm MX is not indicated during lactation.
4.7. Effects on ability to drive and use machines
None known.
4.8 Undesirable effects
Adverse drug reactions from multiple sources including clinical trials and post-marketing experience (Table 1) are listed according to the system organ class in MedDRA. Within each system organ class, the adverse drug reactions are ranked by frequency, the most frequent first. Within each frequency grouping, adverse drug reactions are presented in the order of decreasing seriousness. In addition the corresponding frequency using the following convention (CIOMS III) is also provided for each adverse drug reaction: very common
(>1/10); common (>1/100, <1/10); uncommon (>1/1,000, <1/100); rare (>1/10,000, <1/1,000); very rare (<1/10,000), and not known (cannot be estimated from the available data).
Table 1
|
Neoplasms benign, malignant and unspecified (including cysts and polyps) | |
|
Uncommon: |
Breast cancer. |
|
Immune system disorders | |
|
Very rare: |
Anaphylactoid reaction. |
|
Not known*: |
Hypersensitivity (incl. anaphylactic reaction and angioedema). |
|
Psychiatric disorders | |
|
Not known*: |
Depression, nervousness, affect lability, libido disorder. |
|
Nervous system disorders | |
|
Common: |
Headache. |
|
Rare: |
Dizziness. |
|
Not known*: |
Migraine. |
|
Vascular disorders | |
|
Very rare: |
Embolism, hypertension, varicose veins (including exacerbation). |
|
Gastrointestinal disorders | |
|
Common: |
Nausea, abdominal pain, abdominal distension. |
|
Not known*: |
Vomiting, diarrhoea. |
|
Hepatobiliary disorders | |
|
V ery rare: Liver function tests abnormal, jaundice cholestatic. Not known*: Cholelithiasis, gallbladder disorder. | |
|
Skin and subcutaneous tissue disorders | |
|
Very rare: |
Contact dermatitis, pigmentation disorders, generalised pruritus, generalised exanthema. |
|
Not known*: |
Alopecia, chloasma, urticaria. |
|
Musculoskeletal and connective tissue disorders | |
|
Rare: |
Pain in extremity (leg pain). |
|
Not known*: |
Back pain. |
|
Reproductive system and breast disorders | |
|
Very common: |
Breast discomfort, breakthrough bleeding. |
|
Not known*: |
Endometrial hyperplasia, uterine leiomyoma, breast pain, breast tenderness, dysmenorrhoea, fibrocystic breast disease, breast enlargement, breast discharge. |
General disorders and administration site conditions
Very common: Application site reactions* *.
Rare: Oedema, weight increased or decreased.
(*) Reported in post-marketing experience.
(**) Application site reactions includes localized bleeding, bruising, burning, discomfort, dryness, eczema, edema, erythema, inflammation, irritation, pain, papules, paraesthesia, pruritus, rash, skin discolouration, skin pigmentation, swelling, urticaria, and vesicles.
Breast cancer risk
• An .up. .to . _2.-.fo.ld_ _increased .risk _ o.f having . breast cancel - diagnosed. _ is. .reported, _ in . .wome.n. taking .combined o.e.strQge.n.-p.roge.stage.n. therapy .for. mo.re than. .5. years...
• Any. increased .risk in. users. of. .oe.strogen-.only.the.rapy. is . substantially. l.ower.than that seen in. users . of. oestrogen-progestagen, .combinations.
• The. .level. of. risk .is. dependent. on .the. duration. of. .use. .(se.e. section. 4.4).
• Results . ..of ..the. ..largest ..randomised ..placebo-controlled .trial. ..(WHI-study). ..and. ..largest epidemiological study. (MWS)..are. presented.
Million. Women, .study- Estimated. additional. .risk . o.f. b.re.a.st. .cance.r . after. .5. years', .use
|
Age range. (years) |
Additional cases |
Risk ratio & |
Additional cases per 1000 |
|
per 1000 never-users of HRT over a. 5. ye.ar period* |
95%CI# |
HRT users over 5 years (95%CI) | |
|
Oestrogen, only. HRT | |||
|
50-65 |
9-12 |
1.2 |
1-2 (0-3) |
|
Combined .o_e_str_oge_n_-p_roge_st_age_n | |||
|
50-65 |
9-12 |
1.7 |
6 .(5-7) |
|
#Overall risk ratio. The risk ratio is not constant but will increase with increasing duration on | |||
|
use Note: Since the background incidence of breast cancer differs by EU country, the number of | |||
|
additional cases of breast cancer will also change proportionately. | |||
|
* Taken from baseline incidence rate in developed countries | |||
US WHI .studies - .additional. risk .of .b.re.a.st cancer..after..5. years'. use.
|
Age .range (yrs) |
Incidence per 1000 women in placebo arm over 5 years |
Risk ratio & 95%CI |
Additional cases per 1000 HRT users over 5 years (95%CI) |
|
CEE oestrogen-only | |||
|
50-79 |
.2.1 |
0.8 (0.7 - 1.0) |
-4(-.6..-.0).*. |
|
CEE+MPA oestrogen & progestagenj | |||
|
50-79 |
.1.7 |
1.2 (1.0 - 1.5) |
+4. (0. -. 9). |
.{When the .analysis, was _restricted to. women who had not used. HRT .prior to.the study there was. no . increased risk. apparent. during.the. .first .5 .years of.tre.atmentJ. after. 5. years.the. .risk was. higher than in non.-users.
*. WHI .study .in .women . with no. .uterus, .which. did . not .show .an increase .in .risk p.fbreast cancer.
Endometrial cancer risk
Postmenopausal. women. with . a. .uterus
The. endometrial, cancer risk .is. about .5 .in .every. 1000 .women .with. .a. uterus .not using HRT..
In. women, .with, a . uterus, use. of oe strogen-only. HRT . is. not. recomm.end_e_d_ .because . it . increases the .risk .of .endometrial .cancer .(see . section .4,4),
Depending. on. the. duration. of oestrogen-only..use . and . oestrogen . dose,. the. increase . in. risk. of endo.metrial _ cancer , in. . epidemiology . studies. _ varied . .from. , between. .5. _ and . .55 . extra. . cases diagnosed, .in .every. 100.0. .women .between the. ages .of. 50 .and. 65,
Adding .a. progestagen .to . oestrogen-only .therapy. for .at .least .12. days. per. cycle. can .prevent. this increased .risk, .In .the . Million .Women .Study .the use . of .five, years, .of combined. .(sequential. or continuous). HRT .did. not .increase. risk _p_fend_pmetrial. cancer .(RR. of 1,0. (0,8.-1,2)),
Ovarian cancer
Lo.ng.-te.rm _ .use. . of., pestrog_en-pnly ., and .. combined. _ .oestrogen-progestagen . .HRT . has ., been, associated . with. .a. . slightly. .increased. .risk . of ovarian . .cancer, . In . the. .Million. .Women. . Study. .5 years. of HRT. resulted. in .1 .extra, cas.e.p.e.r .250.0. users,
Risk of venous thromboembolism
HRT ...is. __ associated ...with ...a. ...i,3_-_3_-fold_ ...increased ..relative. ...risk ...of...developing ...venous thrpmbp.e.mb.oli.S.m_ XYTE), .i,e, deep. .vein. .thrombosis, .or .pulmonary, .embolism, .The. .occurrence, of such . an .event. is . more. . likely, .in . the. . first. year. of .using . HT. .(see. .section .4,,4), . Results, .of.the whi .studies, are. pre s.ente.d;.
WHI Studies. - Additional .risk of VTE. over. 5 years'. use
|
Age. .range. (years). |
Incidence per 1000 women in placebo arm over 5 years |
Risk ratio and 95%CI |
Additional cases per 1000 HRT users |
|
Oral oestrogen-only* | |||
|
50-59 |
7 |
1,2 (0,6-2,4) |
1 (-3 - 10) |
|
Oral . combined . pestrog_e_n-proge_stagen | |||
|
50-59 |
4 |
2,3 (1,2 - 4,3) |
.5.a.-..i3). |
* Study_ in women with no_ uterus.
Risk of coronary artery disease
• The. .risk .of. coronary .artery .disease . is . slightly. .increased . in. users. _pf_cp.mbined. oe.str.ogen-. progestagen. .HRT. over .the .age. .of. 60 .(see .section .4,4).-.
Risk of ischaemic stroke
• The. use. of .oestrogen-only, and .oestrogen, .and. progestagen .therapy . is. .associated. with . an up. to. 1,5. ibid. .increased . relative, .risk .ofis-chae-mic. .stroke,, .The .risk _pfbae.morrh_agi.c . stroke, .is not. increased, during, use. of hrt,
• This, relative .risk is. not dependent _on. age. or on. .duration _ of use, but as the baseline _risk, is
strongly. . age-depe.nde.nt, .the. overall . risk . of .stroke. in. .women . who . use. HRT. will. .increase with . age, see . section .4.4,
WHI studies combined - .Additional, risk of ischaemic stroke* over 5 years' use
|
Age range (years) |
Incidence per 1000 women in placebo arm over 5 years |
Risk ratio and 95%CI |
Additional cases per 1000 HRT users over 5 years |
|
50-59 |
8 |
1__3. (_1_1.1.6) |
3.a-5t |
*. No. .differentiation, .was. made, between, ischaemic .and. haemorrhagic. stroke.
The following other adverse reactions have been reported in association with oestrogen alone and-oestrogen-progestagen treatments:
• Cerebrovascular accident
• Skin and subcutaneous disorders: chloasma, erythema multiforme, erythema nodosum, vascular purpura
• Gall bladder disease
• Probable dementia over the age of 65 (see section 4.4)
• Dry eyes
• Tear film composition changes
4.9 Overdose
This is not likely due to the mode of administration.
Signs and Symptoms: Signs of acute oestrogen overdosage may be either one of, or a combination of, breast discomfort, fluid retention and bloating or nausea.
Treatment: Overdosage can if necessary be reversed by removal of the patch(es).
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: oestrogens ATC code G 03 C A 03.
The active ingredient, synthetic 17P-estradiol is chemically and biologically identical to endogenous human estradiol. It substitutes for the loss of oestrogen production in menopausal women, and alleviates menopausal symptoms.
5.2 Pharmacokinetic properties
Absorption
Steady-state serum oestradiol concentrations are reached within 8 hours after application of Estraderm MX 50 to the skin, and remain stable during 4 days. The mean E2 concentration during steady-state of Estraderm MX 50 is 41 pg/mL in healthy postmenopausal women, corresponding to a mean increase of 37 pg/mL over the mean baseline value of 4 pg/mL (range 2.1 to 9.0 pg/mL). The E2:E1 ratio increases from a postmenopausal value of 0.3 to a value of 1.3, similar to the physiological ratio of E2 to E1 observed before the menopause in women with normally functioning ovaries. During continuous treatment of postmenopausal women with Estraderm MX 50 twice weekly for 12 weeks, mean E2 plasma concentrations rise by 36 pg/mL above baseline at the end of the treatment phase, without any indication that accumulation of E2 levels occurs
With Estraderm MX 25, E2 plasma levels half those observed with Estraderm MX 50 are measured, and with Estraderm MX 100 plasma E2 levels are slightly more than double those measured with Estraderm MX 50 .
Plasma oestradiol concentrations return to baseline value within 24 hours after removal of the patch.
Distribution
In plasma, oestradiol is largely bound to sex hormone binding globulin (SHBG) and albumin. Only a fraction is free and biologically active.
Metabolism
Transdermally applied oestradiol is metabolised in the same way as the endogenous hormone. Oestradiol is metabolised to oestrone, then later -primarily in the liver - to oestriol, epioestriol and catechol oestrogens, which are then conjugated to sulphates and glucoronides. Cytochrome 450 isoforms CYP1A2 and CYP3A4 catalyse the hydroxylation of oestradiol forming oestriol. Oestriol is glucuronidated by UGT1A1 and UGT2B7 in humans. Metabolic plasma clearance ranges from 650 to 900 L/(day x m2). Oestradiol metabolites are also subject to enterohepatic circulation. Oestradiol metabolites are far less active than oestradiol.
Elimination
Oestradiol and its metabolites are mainly excreted in the urine. The plasma elimination half-life of oestradiol is about 1 hour. Oestradiol conjugates
excreted in the urine return to pre-application levels on the second or third day after removal of the system.
5.3 Preclinical safety data
Animal studies with oestradiol have only shown effects which can be expected from an estrogenic substance.
Acute toxicity of oestrogens is low. Because of marked differences between animal species and between animals and humans, preclinical results possess a limited predictive value for the application of oestrogens in humans.
In experimental animals oestradiol displayed an embryolethal effect at relatively low doses; malformations of the urogenital tract and feminisation of male foetuses were observed.
Long-term, continuous administration of natural and synthetic oestrogens in certain animal species increases the frequency of carcinomas of the mammary gland, uterus, cervix, vagina, testis, and liver.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Acrylate, methacrylate, isopropyl palmitate, polyethylene terephthalate, ethylenevinylacetate copolymer, silicone-coating (on the inner side of the protective release liner which is removed before patch application).
6.2. Incompatibilities
None known
6.3.
Shelf Life
6.4. Special Precautions for Storage
Store below 25°C.
Keep out of the reach of children both before and after use.
6.5. Nature and Contents of Container
Each system is individually heat sealed in a paper/aluminium/polyethylene foil pouch. Eight or twenty four Estraderm MX pouches are placed in an appropriately sized carton which comprises the finished product (one or three month's treatment respectively).
6.6 Special precautions for disposal
See Section 4.2. Exposure of Estraderm MX patches to ultra-violet light results in degradation of oestradiol. Patches should not be exposed to sunlight. They should be applied immediately after removal from the pouch to skin sites covered by clothing.
After use, the Estraderm MX patch should be folded (adhesive surfaces pressed together) and discarded in such a way as to keep them out of the reach and sight of children.
7. MARKETING AUTHORISATION HOLDER
Novartis Pharmaceuticals UK Ltd Trading as Ciba Laboratories Frimley Business Park Frimley Camberley
Surrey GU16 7SR
8 MARKETING AUTHORISATION NUMBER
Estraderm MX 25: PL 00101/0486
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
10/02/2009