Estring 7.5 Microgram/24 Hours Vaginal Delivery System
Out of date information, search anotherPACKAGE LEAFLET: INFORMATION FOR THE USER

Estradiol Hemihydrate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, please ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If any of the side effects gets serious, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Estring is and what it is used for
2. What you need to know before you use Estring
3. How to use Estring
4. Possible side effects
5. How to store Estring
6. Contents of the pack and other information
1. What Estring is and what it is used for
Estring vaginal delivery system is a vaginal ring which contains the active ingredient estradiol hemihydrate, which is a naturally occurring form of the main female sex hormone, oestrogen.
Women’s ovaries gradually produce less oestrogen as they approach menopausal age (also referred to as “the change"). Low levels of oestrogen can cause symptoms such as vaginal dryness, inflammation or itching, and this in turn can lead to sore or painful sexual intercourse, and an increased susceptibility to vaginal or urinary infections.
Estring vaginal delivery system is part of hormone replacement therapy (HRT) that acts locally in the vagina to maintain the adequate levels of oestrogen to relieve these symptoms in post menopausal women. It does not treat other symptoms of menopause such as hot flushes and sweats. Tell your doctor if you also have these problems.
2. What you need to know before you use Estring
Medical check-up
Estring may not be suitable for all women. Before you start using Estring, your doctor should ask about your own and your family’s medical history. Your doctor may decide to examine your breasts and/ or your abdomen, and may do an internal examination - but only if these examinations are
2
necessary for you, or if you have any special concerns.
Once you’ve started on HRT, it is recommended that you see your doctor for regular check-ups (at least once a year). At these check-ups, your doctor may discuss with you the benefits and risks of continuing to take HRT.
Be sure to:
• go for regular breast screening and cervical smear tests
• regularly check your breasts for any changes such as dimpling of the skin, changes in the nipple, or any lumps you can see or feel
Do not use Estring if you have the following conditions:
• if you are allergic to estradiol or similar medicines for hormone replacement therapy, or any of the other ingredients of this medicine (listed in Section 6)
• hereditary blood disorder (porphyria)
• cancer that is sensitive to oestrogen e.g. endometrial cancer (cancer of the womb)
• previous or current history of an ‘embolus’ (blood clots that travel through the bloodstream and block blood vessels in the leg or elsewhere, also called ‘deep vein thrombosis’ (DVT))
• previous or current history of breast cancer
• genital bleeding which you have not made your doctor aware of
• previous or recent liver disease where liver function tests are still abnormal
• overgrown lining of your womb (untreated endometrial hyperplasia)
• previous or a present case of blocked arteries that could cause cardiovascular diseases like angina or a heart attack
• blood clotting disorder (thrombophilic disorder such as protein C, protein S or antithrombin deficiency).
Warnings and precautions
Talk to your doctor or pharmacist before using Estring
Your doctor will assess your health and discuss the risk and benefits of hormone replacement therapy carefully before prescribing Estring to you. Tell your doctor if you currently have or have had in the past any of the following conditions to help them decide if you will be suitable for treatment with Estring:
• A prolapse (weakening of the structures supporting your internal organs) or have ever had an operation for prolapse
• You are on long term steroid therapy or have problems with your adrenal glands such as a disease called Cushing’s disease (where you may experience thinning or reddening of the skin)
• Vaginal discomfort, bleeding or pain in your vagina including irritation or discharge which may be due to ulcers or infection
• If you have a short narrow vagina from previous surgery or the effect of a condition called vaginal atrophy
• Kidney or heart disease
• High levels of triglycerides (a type of fat) in your blood
• Liver diseases (including liver cancers)
• Diabetes
• History of cancer (particularly breast cancer) in your family
• Risk factors for blood clots in the veins (venous thromboembolism or deep vein thrombosis), see later in Section 2 where the risks of taking Estring are described
• High blood pressure
• Migraine or severe headache
• Uterine fibroids (growth on the walls of the womb)
• Fits (epilepsy)
• Gallstones
• Autoimmune disease, systemic lupus erythematosus (SLE)
• History of endometrial hyperplasia (an increase in the number of cells of the inner lining of the womb)
• Endometriosis (inner lining tissues of the womb found in places other than the womb)
• Problems with your hearing caused by scarring in the ear (otosclerosis)
• Asthma.
If any of the above conditions get worse or come back while you are using Estring you should remove Estring vaginal delivery system and see your doctor straight away.
Safety of HRT
As well as benefits, HRT has some risks which you need to consider when you’re deciding whether to take it, or whether to carry on taking it. These risks are taken from HRT medicines which circulate in the blood and it is not known how these risks apply to local treatments such as Estring.
Effects on your heart or circulation
Heart Disease
HRT is not recommended for women who have heart disease, or have had heart disease recently. If you have ever had heart disease, talk to your doctor to see if you should be taking HRT.
HRT will not help to prevent heart disease.
Studies with one type of HRT (containing combined oestrogen-progestogen HRT) have shown that women over the age of 60 may be slightly more likely to get heart disease. For other types of HRT, the risk is likely to be similar, although this is not yet certain.
If you get:
• a pain in your chest that spreads to your arm or neck See a doctor as soon as possible and do not take any more HRT until your doctor says you can. This pain could be a sign of heart disease.
Stroke
Recent research suggests that HRT slightly increases the risk of having a stroke. Other things that can increase the risk of stroke include:
• getting older
• high blood pressure
• smoking
• drinking too much alcohol
• an irregular heartbeat
If you are worried about any of these things, or if you have had a stroke in the past, talk to your doctor to see if you should take HRT.
Looking at women in their 50s who are not taking HRT - on average, over a 5-year period, 8 in 1000 would be expected to have a stroke.
For women in their 50s who are taking HRT, the figure would be 11 in 1000, over a 5-year period.
If you get:
• unexplained migraine-type headaches, with or without disturbed vision
See a doctor as soon as possible and do not take any more
HRT until your doctor says you can. These headaches may be an early warning sign of a stroke.
Blood Clots
HRT may increase the risk of blood clots in the veins (also called deep vein thrombosis, or DVT), especially during the first year of taking it.
These blood clots are not always serious, but if one travels to the lungs, it can cause chest pain, breathlessness, collapse or even death. This condition is called pulmonary embolism, or PE.
DVT and PE are examples of a condition called venous thromboembolism, or VTE.
You are more likely to get a blood clot:
• if you are seriously overweight
• if you have had a blood clot before
• if any of your close family have had blood clots
• if you have had one or more miscarriages
• if you have any blood clotting problem that needs treatment with a medicine such as warfarin
• if you’re off your feet for a long time because of major surgery, injury or illness
• if you have a rare condition called SLE.
If any of these things apply to you, talk to your doctor to see if you
should take HRT.
Looking at women in their 50s who are not taking HRT - on average, over a 5-year period, 7 in 1000 would be expected to get a blood clot.
For women in their 50s who are taking HRT, the figure would be 8 in 1000, over a 5-year period.
If you get:
• painful swelling in your leg
• sudden chest pain
• difficulty breathing
See a doctor as soon as possible and do not take any more
HRT until your doctor says you can. These may be signs of a blood clot. If you’re going to have surgery, make sure your doctor knows about it. You may need to stop taking HRT about 4 to 6 weeks before the operation, to reduce the risk of a blood clot. Your doctor will tell you when you can start taking HRT again.
Effects on your risk of developing cancer Breast Cancer
Women who have breast cancer, or have had breast cancer in the past, should not take HRT.
Taking HRT slightly increases the risk of breast cancer; so does having a later menopause. The risk for a post-menopausal woman taking oestrogen-only HRT for 5 years is about the same as for a woman of the same age who is still having periods over that time and not taking HRT.
For all kinds of HRT, the extra risk of breast cancer goes up the longer you take it, but returns to normal within about 5 years after stopping HRT.
Your risk of breast cancer is also higher:
• if you have a close relative (mother, sister or grandmother) who has had breast cancer
• if you are seriously overweight
Looking at women aged 50 to 65 who are not taking HRT - on
average, over a 5-year period, 9-12 in 1000 would be expected to develop breast cancer.
For women aged 50 to 65 who are taking HRT, the figure would be 10-14 in 1000, over a 5-year period.
Endometrial Cancer (cancer of the lining of the womb) Taking oestrogen-only HRT tablets for a long time can increase the risk of cancer of the lining of the womb (the endometrium). It is possible there may be a similar risk with oestrogen cream/rings used directly in the vagina for repeated treatments or over a long time.
If you get:
• Breakthrough bleeding or spotting, it’s usually nothing to worry about, but you should make an appointment to see your doctor. It could be a sign that your endometrium has become thicker.
Ovarian Cancer
Ovarian cancer (cancer of the ovaries) is very rare, but it is serious. It can be difficult to diagnose, because there are often no obvious signs of the disease.
Some studies have indicated that taking oestrogen-only HRT for more than 5 years may slightly increase the risk of ovarian cancer. It is not yet known whether other kinds of HRT increase the risk in the same way.
Other information
HRT will not prevent memory loss. In one study of women who started using combined or oestrogen-only HRT after the age of 65, a small increase in the risk of dementia was observed.
Women with hypertriglyceridaemia may experience large increases of their plasma triglycerides, which can lead to inflammation of the pancreas (pancreatitis). Symptoms of pancreatitis may include abdominal pain, abdominal swelling, fever and feeling or being sick.
Blood tests
If you are about to have any blood tests, (e.g. to test for presence of an excess of triglycerides (a type of fat found in the blood), you must tell your doctor that you are using Estring vaginal delivery system, as these tests can be affected by use of the vaginal delivery system.
Other medicines and Estring
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
In particular, tell your doctor if you are taking:
• Anticonvulsants used in the treatment of epilepsy such as phenobarbitol, phenytoin, or carbamazepine
• Anti-infectives such as rifampicin, rifabutin
• Drugs used to treat HIV such as ritonavir, nelfinavir, nevirapine, or efavirenz
• Herbal preparations containing St. John’s wort (Hypericum perforatum).
It is recommended that the ring is removed when constipated or using vaginal preparations.
Pregnancy and breast-feeding
Estring should not be used during pregnancy. If you become pregnant whilst using Estring, you should stop using Estring immediately and tell your doctor that you are pregnant.
Estring should not be used whilst breast-feeding. Ask your doctor or pharmacist for advice before taking any medicine while breast-feeding.
Driving and using machines
There are no special precautions, you can drive or operate machinery as long as you feel well.
3. How to use Estring
Always use this medicine exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure. Please follow the instructions below carefully. You should wash your hands thoroughly before inserting Estring vaginal delivery system.
To insert Estring vaginal delivery system into your vagina
• Relax and find a position which feels comfortable for you.
• Either stand with one foot on a chair or lie on your back with your knees bent up.
• With one hand, open the folds of skin around the vagina.
• With the other hand, press the ring into an oval.
• Push the ring into your vagina as far as it will go - upwards and backwards towards the small of the back.
• Finally, wash your hands.
If the ring falls out, it should be rinsed in lukewarm (not hot) water and then reinserted.
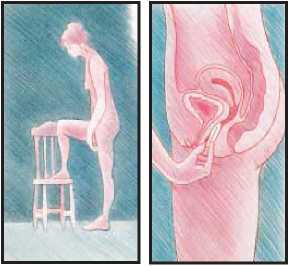
While using Estring vaginal delivery system
You may be aware of the ring at first but this feeling should go away. The ring may take several weeks to have the full effect. As the ring begins to work you may notice an increase in vaginal lubrication (wetness), this is normal and should be the same as you experienced before the menopause.
Most women and their partners have found it acceptable for the ring to stay in place during sexual intercourse. If you or your partner finds the ring uncomfortable or unacceptable it may be removed.
The ring may move down in the vagina and become noticeable during straining to empty your bowels. If this happens the ring can be easily pushed back into position with your finger.
If you know that you are constipated or need to strain to empty your bowels then you should remove the ring first.
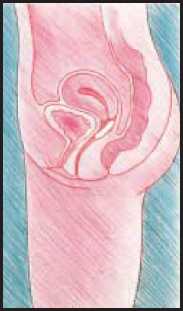
To take out Estring vaginal delivery system from your vagina
• Relax and find a position which feels comfortable for you.
• Either stand with one foot on a chair or lie on your back with your knees bent up.
• With one hand, open the folds of skin around the vagina.
• With the other hand, hook your finger around the ring.
• Pull the ring gently downwards and forward.
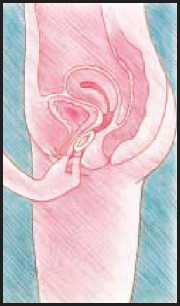
• Finally, wash your hands.
How long you should use your ring for
Each ring should be worn continuously for 3 months, and then replaced by a new ring, as appropriate. The maximum recommended duration of continuous therapy is two years. Your doctor will try to give you the lowest effective dose possible, and HRT should only be continued as long as the benefit in relief of severe symptoms outweighs the risk.
Routine examinations whilst using Estring vaginal delivery system
It is recommended that you have regular screening through the National Breast Cancer Screening Programme and National Cervical Cancer Screening Programme. Your doctor can provide details. You are also recommended to report any changes in your breasts to your doctor as soon as possible.
If you stop using your ring
Your symptoms may return after about 3 weeks.
Use in children
Estring vaginal delivery system is not recommended for use in children.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Remove Estring vaginal delivery system and see your doctor straight away if you develop any of the conditions listed under ‘Do not use Estring’ or have any of the following:
• Symptoms of an allergic reaction. This may include skin rash, hives, itching.
• jaundice (yellowing of the skin and whites of the eyes)
• significant increase in blood pressure
• migraine-type headache
• if you become pregnant
• painful swelling of the leg
• sudden pain in the chest
• shortness of breath (dyspnoea)
• persistent or severe vaginal discomfort, ulceration or swelling after the ring has been inserted.
If any of the following side effects gets serious, or if you notice any side effects not listed in this leaflet, tell your doctor:
Common side effects may effect up to 1 in 10 people using the Estring vaginal delivery system:
• Urinary tract infection
• Infection and itching inside and around the vagina
• Discomfort/pain in the stomach area (abdomen)
• Any persistent feeling of the ring in the vagina or pressing on the bladder/rectum (back passage)
• Pain on passing urine
• Generalised itching
• Increased sweating
The symptoms mentioned above occur more frequently in untreated post-menopausal women.
Other side effects reported during treatment of patients with other forms of oestrogen therapy include:
• abdominal uterine bleeding such as breakthrough bleeding or spotting, changes in menstrual flow, vaginal inflammation and vaginal discharge
• a tendency to get thrush
• breast pain, breast tenderness, swollen breasts, discharge from the nipples
• feeling sick, a feeling of being bloated, abdominal pain
• headache or migraine
• dizziness
• changes in mood including anxiety and depression
• joint pain, leg cramps
• changes in your interest in sex (increased or decreased libido)
• visible swelling of the face or ankles
• rash, itchiness and dark or red patches on the skin
• changes in hair growth (loss or increase)
• difficulty wearing contact lenses
• changes in weight (increase or decrease)
• changes in your triglyceride levels (fatty substances in the blood)
• memory loss (dementia)
• gallbladder disease (e.g. gallstones)
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse.
This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/vellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Estring
Do not store above 25°C.
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and also on the foil pouch after EXP. The expiry date refers to the last day of that month.
Do not use Estring vaginal delivery system if it is discoloured, misshapen, or does not have a smooth surface.
Used rings still contain some of the active hormonal ingredient. The used ring should be placed within the original pouch or in a plastic bag, then sealed and discarded safely, out of the reach and sight of children.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What Estring contains
- The active substance in Estring is estradiol hemihydrate 2 mg, corresponding to 1.94 mg estradiol.
- The other ingredients are silicone fluid, barium sulphate, silicone
elastomer Q7-4735A, silicone elastomer Q7-4735B.
20
What Estring looks like and contents of the pack
Estring vaginal ring is a slightly opaque ring made of a silicone elastomer, with a whitish core, containing a drug reservoir of the active ingredient, estradiol hemihydrate.
Estring is individually packed in a heat-sealed rectangular pouch consisting of, from outside to inside: Polyester/Aluminium foil/Low density Polyethylene. Each pouch is provided with a tear-off notch on one side and is packed into a cardboard carton.
Marketing Authorisation Holder and Manufacturer
The manufacturing authorisation holder is Pfizer Limited, Ramsgate Road, Sandwich, Kent, CT13 9NJ, UK.
The manufacturer is QPharma AB, Agneslundsvagen, 27, SE-201 25, Malmo, Sweden.
Company Contact address
For further information on this medicine, please contact Medical Information at Pfizer Limited in Walton Oaks, Tadworth, Surrey.
Tel: +44 1304 616161.
This medicinal product is authorised in the Member States of the EEA under the following names:
Spain, France, Italy, Luxembourg, Slovenia, Belgium, UK: Estring
This leaflet was last revised in 09/2013.
Ref: EG 2_0


2012-0010976/2

23