Fanhdi 50 I.u./Ml Powder And Solvent For Solution For Injection
Out of date information, search anotherSUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Fanhdi® 50 I.U./ml powder and solvent for solution for injection.
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Human coagulation factor VIII, Ph. Eur.
Fanhdi® is presented as a lyophilised powder for solution for injection containing nominally 250, 500, 1000 or 1500 I.U. human coagulation factor VIII per vial.
The product contains approximately 25, 50 or 100 I.U./ml of human coagulation factor VIII when reconstituted with 10 ml of Water for Injections for the presentations of 250, 500 and 1000 I.U. The presentation of 1500 I.U. is reconstituted with 15 ml of Water for Injections and contains approximately 100 I.U./ml.
The factor VIII:C potency (I.U.) is determined using the European Pharmacopoeia chromogenic assay. The specific activity of Fanhdi® is at least 2.5 to 10 I.U. factor VIII:C/mg protein depending on its strength (250, 500, 1000 or 1500 I.U.).
For a full list of excipients, see section 6.1.
3. PHARMACEUTICAL FORM
Powder and solvent for solution for injection.
Vial containing white or pale yellow powder and syringe with Water for Injections (solvent).
4. CLINICAL PARTICULARS
4.1. Therapeutic indications
Fanhdi® is indicated for the treatment and prophylaxis of bleeding in patients with haemophilia A (congenital factor VIII deficiency).
Despite the von Willebrand factor content and functionality of this product there are no data from clinical trials supporting use in von Willebrand disease.
This product may be used in the management of acquired factor VIII deficiency.
4.2. Posology and method of administration
Treatment should be initiated under the supervision of a physician experienced in the treatment of haemophilia.
Posology
The dosage and duration of the substitution therapy depend on the severity of the factor VIII deficiency, on the location and extent of the bleeding and on the patient’s clinical condition.
On demand treatment
The number of units of factor VIII administered is expressed in International Units (I.U.), which are related to the current WHO standard for factor VIII products. Factor VIII activity in plasma is expressed either as a percentage (relative to normal human plasma) or in International Units (relative to an International Standard for factor VIII in plasma).
One International Unit (I.U.) of factor VIII activity is equivalent to that quantity of factor VIII in one ml of normal human plasma. The calculation of the required dosage of factor VIII is based on the empirical finding that 1 International Unit (I.U.) factor VIII per kg body weight raises the plasma factor VIII activity by 2.1 ± 0.4% of normal activity. The required dosage is determined using the following formula:
Required units = body weight (kg) x desired factor VIII rise (%) (I.U./dl) x 0.5
The amount to be administered and the frequency of administration should always be oriented to the clinical effectiveness in the individual case.
In the case of the following haemorrhagic events, the factor VIII activity should not fall below the given plasma activity level (in % of normal or I.U./dl) in the corresponding period. The following table can be used to guide dosing in bleeding episodes and surgery:
Degree of haemorrhage/ Factor VIII level Frequency of doses (hours)/
Type of surgical required (%) Duration of therapy (days)
procedure_(I.U./dl)_
Haemorrhage
Repeat every 12 to 24 hours. At least 1 day, until the bleeding episode as indicated by pain is resolved or healing is achieved.
Repeat infusion every 12-24 hours for 3-4 days or more until pain and acute disability are resolved.
Repeat infusion every 8 to 24 hours until threat is resolved.
Early haemarthrosis, 20 - 40
muscle bleeding or oral
bleeding
More extensive 30 - 60
haemarthrosis, muscle bleeding or haematoma
Life threatening 60 - 100
haemorrhages
Minor
including tooth extraction
Major
30 - 60 Every 24 hours, at least 1 day, until
healing is achieved.
80 - 100 (pre-and postoperative)
Repeat infusion every 8-24 hours until adequate wound healing, then therapy for at least another 7 days to maintain a factor VIII activity of 30% to 60% (I.U./dl).
Prophylaxis
For long term prophylaxis against bleeding in patients with severe haemophilia A, the usual doses are 20 to 40 I.U. of factor VIII per kg body weight at intervals of 2 to 3 days. In some cases, especially in younger patients, shorter dosage intervals or higher doses may be necessary.
Continuous infusion
During the course of treatment, appropriate determination of factor VIII levels is advised to guide the dose to be administered and the frequency of repeated infusions. In the case of major surgical interventions in particular, precise monitoring of the substitution therapy by means of coagulation analysis (plasma factor VIII activity) is indispensable. Individual patients may vary in their response to factor VIII, achieving different levels of in vivo recovery and demonstrating different half-lives.
Patients should be monitored for the development of factor VIII inhibitors. If the expected factor VIII activity plasma levels are not attained, or if bleeding is not controlled with an appropriate dose, an assay should be performed to determine if a factor VIII inhibitor is present. In patients with high levels of inhibitor, factor VIII therapy may not be effective and other therapeutic options should be considered. Management of such patients should be directed by physicians with experience in the care of patients with haemophilia. See section 4.4.
Paediatric population
There are insufficient data from clinical trials to recommend the use of Fanhdi® in children less than 6 years of age.
As the posology is adjusted to the clinical outcome of the above mentioned conditions, the posology in children, by body weight, is not considered to be different to that of adults.
Method of administration
Dissolve the preparation as described in section 6.6. The product should be administered via the intravenous route. Fanhdi® should be administered at a rate of no more than 10 ml/min.
4.3. Contra-indications
Hypersensitivity to the active substance or to any of the excipients.
4.4. Special warnings and precautions for use
As with any intravenous protein product, allergic type hypersensitivity reactions are possible. The product contains traces of human proteins other than factor VIII. Patients should be informed of the early signs of hypersensitivity reactions including hives, generalised urticaria, tightness of the chest, wheezing, hypotension, and anaphylaxis. If these symptoms occur, they should be advised to discontinue use of the product immediately and contact their physician.
In case of shock, the current medical standards for shock-treatment should be observed.
Standard measures to prevent infections resulting from the use of medicinal products prepared from human blood or plasma include selection of donors, screening of individual donations and plasma pools for specific markers of infection and the inclusion of effective manufacturing steps for the inactivation/removal of viruses. Despite this, when medicinal products prepared from human blood or plasma are administered, the possibility of transmitting infective agents cannot be totally excluded. This also applies to unknown or emerging viruses and other pathogens. The measures taken are considered effective for enveloped viruses such as HIV, HBV and HCV. The measures taken may be of limited value against non-enveloped viruses such as HAV and parvovirus B19. Parvovirus B19 infection may be serious for pregnant women (foetal infection) and for individuals with immunodeficiency or increased erythropoiesis (e.g. haemolytic anaemia).
The formation of neutralising antibodies (inhibitors) to factor VIII is a known complication in the management of individuals with haemophilia A. These inhibitors are usually IgG immunoglobulins directed against the factor VIII procoagulant activity, which are quantified in Bethesda Units (BU) per ml of plasma using Nijmegen’s modified assay. The risk of developing inhibitors is correlated to the exposure to anti-haemophilic factor VIII, this risk being highest within the first 20 exposure days. Rarely, inhibitors may develop after the first 100 exposure days. Patients treated with human coagulation factor VIII should be carefully monitored for the development of inhibitors by appropriate clinical observations and laboratory tests. See also section 4.8.
Cases of recurrent inhibitor (low titre) have been observed after switching from one factor VIII product to another in previously treated patients with more than 100 exposure days who have a previous history of inhibitor development. Therefore, it is recommended to monitor patients carefully for inhibitor occurrence following any product switch.
Appropriate vaccination (hepatitis A and B) should be considered for patients in regular receipt of human plasma-derived factor VIII products.
It is strongly recommended that every time that Fanhdi® is administered to a patient, the name and batch number of the product are recorded in order to maintain a link between the patient and the batch of the product.
4.5. Interaction with other medicinal products and other forms of interaction
No interactions of human coagulation factor VIII products with other medicinal products are known.
4.6. Pregnancy and lactation
Animal reproduction studies have not been conducted with factor VIII.
Based on the rare occurrence of haemophilia A in women, experience regarding the use of factor VIII during pregnancy and breast-feeding is not available.
Therefore, factor VIII should be used during pregnancy and lactation only if clearly indicated.
4.7. Effects on ability to drive and use machines
Fanhdi® has no or negligible influence on the ability to drive and use machines.
4.8. Undesirable effects
Hypersensitivity or allergic reactions (which may include angioedema, burning and stinging at the infusion site, chills, flushing, generalised urticaria, headache, hives, hypotension, lethargy, nausea, restlessness, tachycardia, tightness of the chest, tingling, vomiting, wheezing) have been observed infrequently, and may in some cases progress to severe anaphylaxis (including shock).
On rare occasions, fever has been observed.
The adverse drug reactions reported are summarised and categorised according to the MedDRA system organ class in the table below. Within each frequency grouping, undesirable effects are presented in order of decreasing of seriousness. Frequency has been determined using the following criteria:
- very common: >1/10 infusions
- common: >1/100 to <1/10 infusions
- uncommon: >1/1,000 to <1/100 infusions
- rare: >1/10,000 to <1/1,000 infusions
- very rare: <1/10,000, not known (cannot be estimated from the available data.)
|
System Organ Class |
Body System Preferred Term |
ADR frequency evaluation |
|
General disorders and administration site conditions |
Pyrexia |
Rare |
Patients with haemophilia A may develop neutralising antibodies to factor VIII. If such inhibitors occur, the condition will manifest itself as an insufficient clinical response. In such cases, it is recommended that a specialised haemophilia centre be contacted.
There is no experience in previously untreated patients.
For information on transmissible agents’ safety, see section 4.4.
4.9. Overdose
No case of overdose has been reported.
5.1 Pharmacodynamic properties
Pharmacotherapeutic Group: Antihaemorrhagics: blood coagulation factor VIII. ATC code: B02BD02.
In Fanhdi®, factor VIII is presented as a complex with von Willebrand factor.
The factor VIII/von Willebrand factor complex consists of two molecules (factor VIII and von Willebrand factor) with different physiological functions.
When infused into a haemophiliac patient, factor VIII binds to von Willebrand factor in the patient’s circulation.
Activated factor VIII acts as a cofactor for activated factor IX, accelerating the conversion of factor X to activated factor X. Activated factor X converts prothrombin into thrombin. Thrombin then converts fibrinogen into fibrin and a clot can be formed.
Haemophilia A is a sex-linked hereditary disorder of blood coagulation due to decreased levels of factor VIII and results in profuse bleeding into joints, muscles or internal organs, either spontaneously or as a result of accidental or surgical trauma. By replacement therapy the plasma levels of factor VIII are increased, thereby enabling a temporary correction of the factor deficiency and correction of the bleeding tendencies.
In addition to its role as a factor VIII protecting protein, von Willebrand mediates platelet adhesion to sites of vascular injury and plays a role in platelet aggregation.
There are insufficient data from clinical trials in children less than 6 years of age.
Data on Immune Tolerance Induction (ITI) have been collected in paediatric and adult patients with haemophilia A who have developed inhibitors to FVIII. The 57 patients from a retrospective study and 14 patients from prospective studies included a broad spectrum of primary and rescue ITI patients with varying prognoses for achieving immune tolerance. Data show that Fanhdi has been used to induce immune tolerance. In patients where tolerance was achieved, bleeding could be prevented or controlled on either prophylactic or on-demand therapy with a FVIII concentrate.
5.2. Pharmacokinetic properties
Plasma factor VIII activity decreases by a two-phase exponential decay.
The half-life of human factor VIII obtained in the clinical trial carried out with Fanhdi® is 14.18 ± 2.55 hours and the "in vivo" recovery is 105.5 ± 18.5%, which is equivalent to approximately 2.1 ± 0.4 I.U./dl per I.U./kg administered (determinations performed following chromogenic method). Additional pharmacokinetic parameters are: mean residence time (MRT) 19.9 ± 4.1 h, area under curve (AUC) 19.3 ± 3.8 I.U. h/ml and clearance 2.6 ± 0.6 ml/h/kg.
5.3. Preclinical safety data
Human plasma coagulation factor VIII (active ingredient for Fanhdi®) is a normal constituent of the human plasma and acts like the endogenous factor VIII. Single dose toxicity testing is of no relevance since higher doses result in overloading.
Repeated dose toxicity testing in animals is impracticable due to interference with developing antibodies to heterologous protein.
6. PHARMACEUTICAL PARTICULARS
6.1. List of excipients
- Histidine
- Albumin (human)
- Arginine
- Water for Injections (solvent)
6.2. Incompatibilities
Fanhdi® must not be mixed with other medicinal products.
Only the provided infusion set should be used because treatment failure can occur as a consequence of factor VIII adsorption to the internal surfaces of some infusion equipment.
6.3. Shelf-life
3 years.
Chemical and physical in-use stability has been demonstrated for 12 hours at 25 °C. From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are responsibility of the user and would normally not be longer than 24 hours at 2 to 8 °C, unless reconstitution has taken place in controlled and validated aseptic conditions.
6.4. Special precautions for storage
Do not store above 30 °C. Do not freeze.
For storage conditions of the reconstituted medicinal product, see section 6.3.
6.5. Nature and contents of container
Fanhdi® is supplied in type II glass vials containing 250, 500, 1000 or 1500 I.U. of factor VIII (lyophilised) and type I glass pre-filled syringes containing 10 ml for the presentations of 250, 500 and 1000 I.U. or 15 ml for the presentation of 1500 I.U. of Water for Injections (solvent).
The accessories supplied with Fanhdi® for reconstitution and administration of the product are: vial adaptor, filter, 2 alcohol swabs and infusion set).
Not all pack sizes may be marketed.
Pack size: 1 lyophilised vial, 1 pre-filled syringe with solvent and accessories.
6.6. Special precautions for disposal and handling
Do not use after the expiry date shown on the label.
Left-over product must never be kept for later use, nor stored in a refrigerator.
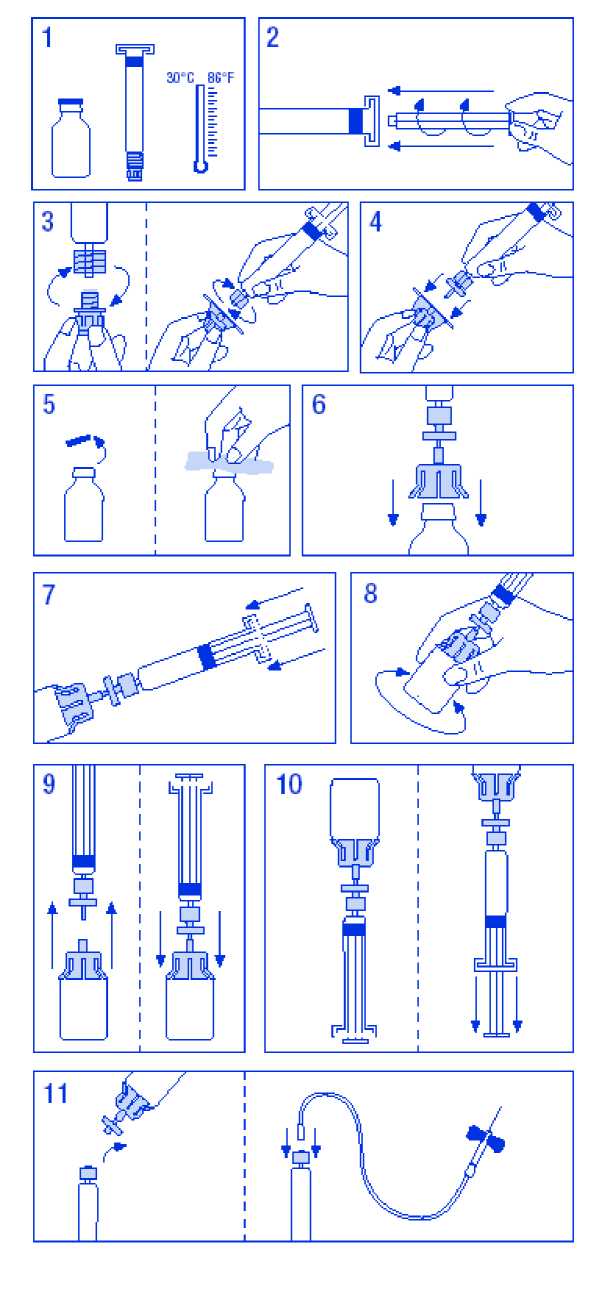
To prepare the solution:
1. Warm the vial and syringe but not above 30°C.
2. Attach plunger to syringe containing diluent.
3. Remove filter from packaging. Remove cap from syringe tip and attach syringe to filter.
4. Remove vial adaptor from packaging and attach to syringe and filter.
5. Remove cap from vial and wipe stopper with swabs provided.
6. Pierce vial stopper with adaptor needle.
7. Transfer all diluent from syringe to vial.
8. Gently shake vial until all product is dissolved. As with other parenteral solutions, do not use if product is not properly dissolved or particles are visible.
9. Briefly separate the syringe/filter from vial/adaptor, to release the vacuum.
10. Invert vial and aspirate solution into syringe.
11. Prepare injection site, separate syringe and inject product using the butterfly needle provided. Injection rate should be 3 ml/min into a vein and never more than 10 ml/min to avoid vasomotor reactions.
Do not re-use administration sets.
Any unused product or waste material should be disposed of in accordance with local requirements.
The solution should be clear or slightly opalescent. Do not use solutions that are cloudy or have deposits.
Reconstituted products should be inspected visually for particulate matter and discolouration prior to administration.
2 - Parets del Valles 08150 Barcelona - SPAIN
Grifols UK Ltd 72 St Andrew’s Road Cambridge CB4 1GS
8 MARKETING AUTHORISATION NUMBER(S)
PL 12930/0004
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
29/02/2000 / 21/06/2005
10 DATE OF REVISION OF THE TEXT
08/08/2014