Femseven 75
Out of date information, search anotherPACKAGE LEAFLET: INFORMATION FOR THE USER
FemSeven® 75, 75 micrograms/24 hours, transdermal patch
Estradiol
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
What is in this leaflet:
1. What FemSeven is and what it is used for
2. What you need to know before you use FemSeven
3. How to use FemSeven
4. Possible side effects
5. How to store FemSeven
6. Contents of the pack and other information
1. WHAT FEMSEVEN IS AND WHAT IT IS USED FOR What is FemSeven?
FemSeven is a Hormone Replacement Therapy (HRT) containing an oestrogen, estradiol hemihydrate, which is a sexual female hormone. FemSeven is used in postmenopausal women more than one year after menopause.
FemSeven is used for:
Relief of symptoms occurring after menopause
During the menopause, the amount of the oestrogen produced by a woman’s body drops. This can cause symptoms such as hot face, neck and chest ("hot flushes"). FemSeven alleviates these symptoms after menopause. You will only be prescribed FemSeven if your symptoms seriously hinder your daily life.
Experience of treatment in women aged over 65 years is limited.
Prevention of osteoporosis
After the menopause some women may develop fragile bones (osteoporosis). You should discuss all available options with your doctor.
If you are an increased risk of fractures due to osteoporosis and other medicines are not suitable for you, you can use FemSeven to prevent osteoporosis after menopause.
Femseven is suitable for women who have undergone a hysterectomy (an operation to remove the womb). If you have not had a hysterectomy, your doctor will normally prescribe another hormone supplement (called a progestogen) for you to use in addition to this one. The progestogen helps to protect the endometrium (lining of the womb). If you have had a hysterectomy because you had endometriosis, your doctor may also prescribe a progestogen, to protect any endometrium left behind.
2. WHAT YOU NEED TO KNOW BEFORE YOU USE FEMSEVEN Medical history and regular check-ups
The use of HRT carries risks which need to be considered when deciding whether to start taking it, or whether to carry on taking it.
The experience treating women with a premature menopause (due to early cessation of ovarian function or ovarian surgery) is limited. If you have a premature menopause the risk of using HRT may be different. Please talk to your doctor.
Before you start (or restart) HRT, your doctor will ask about your own and your family’s medical history. Your doctor may decide to perform a physical examination. This may include an examination of your breasts and/or an internal examination, if necessary.
Once you have started on Femseven you should see your doctor for regular check-ups (at least once a year). At these check-ups, discuss with your doctor the benefits and risks of continuing with Femseven.
Go for regular breast screening, as recommended by your doctor.
Do not use FemSeven
If any of following applies to you. If you are not sure about any of the points below, talk to your doctor before taking Femseven
Do not use Femseven
• If you have or have ever had breast cancer, or if you are suspected of having it.
• If you have cancer which is sensitive to oestrogens, such as cancer of the womb lining (endometrium), or if you are suspected of having it.
• If you have any vaginal bleeding for which the cause is not known.
• If you have excessive thickening of the womb lining (endometrial hyperplasia) that is not being treated.
• If you have or have ever had a blood clot in a vein (thrombosis), such as in the legs (deep venous thrombosis) or the lungs (pulmonary embolism).
• If you have a blood clotting disorder (such as protein C, protein S, or antithrombin deficiency).
• If you have or recently have had a disease caused by blood clots in the arteries, such as a heart attack, stroke or angina.
• If you have or have ever had a liver disease and your liver function tests have not returned to normal.
• If you have an inherited disease (porphyria) characterised by an accumulation of toxic compounds (prophyrins) in the body.
• If you are allergic (hypersensitive) to Estradiol hemihydrate or any of the other ingredients of Femseven. (listed in section “6. Content of the pack and other information”))
If you are not sure of any of the above, consult your doctor before using FemSeven.
If any of the above conditions appear for the first time while using Femseven, stop using it at once and consult your doctor immediately.
Warning and precautions
Talk to your doctor or pharmacist before using FemSeven.
FemSeven is not a contraceptive. As a consequence:
• For women who still have their womb, a treatment with progestogen hormone will be added for the last twelve days of treatment with FemSeven at least.
• If it is less than 12 months since your last menstrual period or you are under 50 years old, you may still need to use additional contraception to prevent pregnancy.
Speak to your doctor for advice.
Before you start the treatment:
Tell your doctor if you have ever had any of the following problems, before you start the treatment, as these may return or become worse during treatment with FemSeven. If so, you should see your doctor more often for check-ups:
• benign tumors of the womb (fibroids inside your womb);
• a growth of womb lining outside your womb (endometriosis);
• a history of abnormal growth of the womb lining (endometrial hyperplasia);
• increased risk of developing blood clots (see “Blood clots in a vein (thrombosis)”);
• an increased risk of getting a cancer which needs estrogens for its development (such as having a mother, sister or grandmother who has had breast cancer);
• high blood pressure;
• a liver disorder, such as a benign liver tumour;
• diabetes;
• gallstones;
• migraine or severe headaches;
• a disease of the immune system that affects many organs of the body (systemic lupus erythematosus);
• epilepsy;
• asthma;
• a disease affecting the eardrum and hearing (otosclerosis);
• a very high level of fat in your blood (triglycerides);
• an increase in the amount of water in your body (fluid retention) due to cardiac or kidney problems.
If you need to have surgery
If you are going to have surgery, tell the surgeon that you are using FemSeven. You may need to stop using FemSeven about 4 to 6 weeks before the operation to reduce the risk of a blood clot (see section 2, Blood clots in a vein). Ask your doctor when you can start using FemSeven again.
Stop using FemSeven and see a doctor immediately
If you notice any of the following when using HRT:
• if you develop any of the conditions mentioned in the “Do not use FemSeven” section
• a yellowing of your skin or the whites of your eyes (jaundice). These may be signs of a liver disease;
• a large rise in your blood pressure (symptoms may be headache, tiredness, dizziness);
• migraine-like headaches which happen for the first time;
• If you become pregnant;
• If you notice a signs of a blood clot, such as:
- painful swelling and redness of the legs;
- sudden chest pain;
- difficulty breathing.
For more information, see “Blood clots in a vein (thrombosis)”
HRT and cancer
Excessive thickening of the lining of the womb (endometrial hyperplasia) and cancer of the lining of the womb (endometrial cancer)
Taking oestrogen-only HRT will increase the risk of excessive thickening of the lining of the womb (endometrial hyperplasia) and cancer of the womb lining (endometrial cancer).
Taking a treatment containing a progestogen in addition to the oestrogen for at least 12 days of each 28 day cycle protects you from this extra risk. So your doctor will prescribe a treatment containing a progestogen separately if you still have your womb. If you have had your womb removed (a hysterectomy), discuss with your doctor whether you can safely take this product without a treatment containing a progestogen.
In women who still have a womb and who are not taking HRT, on average, 5 in 1 000 will be diagnosed with endometrial cancer between the age of 50 and 65.
For women aged 50 to 65 who still have a womb and who take oestrogen-only HRT, between 10 and 60 women in 1 000 will be diagnosed with endometrial cancer (i.e. between 5 and 55 extra cases), depending on the dose and for how long it is taken.
FemSeven contains a higher dose of oestrogens than other oestrogen-only HRT products. The risk of endometrium cancer when using FemSeven together with a treatment containing a progestogen is not known.
Breast cancer:
Evidence suggests that taking combined oestrogen-progestogen and possibly also oestrogen-only HRT increases the risk of breast cancer. The extra risk depends on how long you take HRT. The additional risk becomes clear within a few years. However, it returns to normal within a few years (at most five) after stopping treatment.
For women who have had their womb removed and who are using oestrogen-only HRT for 5 years, little or no increase in breast cancer risk is shown.
Compare
Women aged 50 to 79 who are not taking HRT, on average, 9 to 17 in 1 000 will be diagnosed with breast cancer over a 5-year period. For women aged 50 to 79 who are taking HRT containing both oestrogen and progestogen hormones over 5 years, there will be 13 to 23 cases in 1 000 users (i.e. an extra 4 to 6 cases)
• Regularly check your breasts. See your doctor if you notice any changes such as:
• dimpling of the skin;
• changes in the nipple;
• any lumps you can see or feel.
Ovarian cancer:
in women taking HRT
will be diagnosed with 5 years, there will be
Ovarian cancer is rare. A slightly increased risk of ovarian cancer has been reported for at least 5 to 10 years.
Women aged 50 to 69 who are not taking HRT, on average about 2 women in 1 000 ovarian cancer over a 5 year period. For women who have been taking HRT for between 2 and 3 cases per 1 000 users (i.e. up to 1 extra case)
Effects of HRT on your heart and circulation Blood clots in a vein (thrombosis)
The risk of blood clots in the veins is about 1.3 to 3- times higher in HRT users than in non-users, especially during the first year of taking it.
Blood clots can be serious, and if one travels to the lungs, it can cause chest pain, breathlessness, fainting or even death.
You are more likely to get a blood clot in your veins as you get older and if any of the following applies to you. You will find the signs of a blood clot in the section “Stop using FemSeven and see a doctor immediately”.
Inform your doctor if any of these situations applies to you:
• you are unable to walk for a long time because of major surgery, injury or illness (see also section 2, if you need to have surgery);
• you are seriously overweight (BMI>30 kg/m2);
• you have any blood clotting problem that needs long-term treatment with a medicine used to prevent blood clots;
• if any of your close relatives has ever had a blood clot in the leg, lung or another organ;
• you have an immune system disease that affects many body organs (systemic lupus erythematosus);
• you have cancer.
Compare
Looking at women in their 50s who are not taking HRT, on average, over a 5-year period, 4 to 7 in 1000 would be expected to get a blood clot in a vein.
For women in their 50s who have been taking HRT containing both oestrogen-progestogen hormones for over 5 years, there will be 9 to 12 cases in 1 000 users (i.e. an extra 5 cases).
For women in their 50s who have had their womb removed and have been taking oestrogen-only HRT for over 5 years, there will be 5 to 8 cases in 1 000 users (i.e. 1 extra case)
Heart disease (heart attack)
There is no evidence that HRT will prevent a heart attack.
Women over the age of 60 years who use HRT containing both oestrogen-progestogen hormones are slightly more likely to develop heart disease than those not taking any HRT.
For women who have had their womb removed and are taking oestrogen-only therapy there is no increased risk of developing heart disease.
Stroke
The risk of getting stroke is about 1.5-times higher in HRT users than in non-users. The number of extra cases of stroke due to use of HRT will increase with age.
Compare
Looking at women in their 50s who are not taking HRT, on average, 8 in 1 000 would be expected to have a stroke over a 5-year period. For women in their 50s who are taking HRT, there will be 11 cases in 1 000 users, over 5 years (i.e. an extra 3 cases).
Other conditions
• HRT will not prevent memory loss. There is some evidence of a higher risk of memory loss in women who start using HRT after the age of 65. Speak to your Doctor for advice.
Other medicines and FemSeven
Some medicines may interfere with the effect of FemSeven. This might lead to irregular bleeding. This applies to the following medicines:
• Medicines for epilepsy (such as phenobarbital, phenytoin and carbamazepin);
• Medicines for tuberculosis (such as rifampicin, rifabutin);
• Medicines for HIV infection (such as nevirapine, efavirenz, ritonavir and nelfinavir);
• Herbal remedies containing St John’s Wort (Hypericum perforatum).
Please tell your doctor or pharmacist if you are using, have recently used or might use any other medicines including medicines obtained without a prescription, herbal medicines or other natural products.
Laboratory tests
If you need a blood test, tell your doctor or the laboratory staff that you are using FemSeven, because this medicine can affect the results of some tests.
Pregnancy and breast-feeding
FemSeven is for use in postmenopausal women only. If you become pregnant, stop using FemSeven and contact your doctor.
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby ask your doctor or pharmacist for advice before taking this medicine.
Dosage
FemSeven is an oestrogen-only patch that should be applied to the skin once weekly on a continuous basis, i.e. each patch is replaced with a new one after 7 days.
Always use FemSeven exactly as your doctor has told you. Check with your doctor or pharmacist if you are not sure.
Children: FemSeven is not recommended for use in children.
Adults and the elderly: Your doctor will aim to prescribe the lowest dose to treat your symptom for as short as necessary. Speak to your doctor if you think this dose is too strong or not strong enough.
You can normally start treatment on any convenient day, unless you still have your womb and are changing to FemSeven from a sequential HRT product. In that case, you should start FemSeven straight after your withdrawal bleed has ended. Each patch is worn for 7 days and replaced with a new one on the next day so that you are always wearing a patch. You will normally wear one patch at a time.
Method of administration
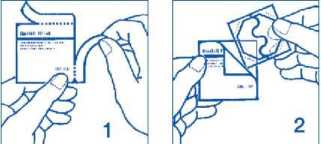
Putting on the patch:
1. Remove the patch from its pouch as shown in pictures 1 and 2.
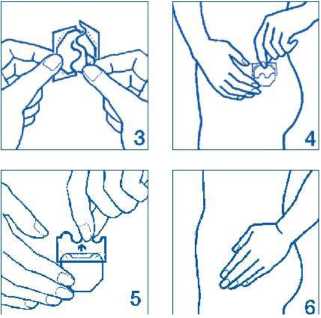
2. Peel off half the protective liner at the ‘S-shaped notch and apply the patch to the skin as in pictures 3 and 4.
Avoid touching the adhesive side of the patch with your fingers as this may prevent it sticking properly later on.
3. Remove the other half of the protective liner then press the patch against your skin with the palm of your hand for at least 30 seconds shown in pictures 5 and 6. The warmth of your body will make the patch stick better.
Choose a place where there is least wrinkling of the skin and where it will not be rubbed off by clothing, for example on your buttocks, hips or abdomen (avoid the waist).
Do not put a patch on or near your breasts.
Do not put a new patch on the same area of skin as the one you have just removed. Make sure the area of skin you use is clean and dry, and not broken or irritated.
If you have applied the patch properly there is little risk of it coming off when you take a bath, shower or swim. You should not expose the patch to sunlight.
To remove the FemSeven patch simply lift off one edge and pull. Fold the patch in half (adhesive against adhesive) and throw it away. If the patch starts to come off before 7 days are up, you should take it off completely and apply a new patch.
Replace it when you would normally have done. If you forget to change your patch at the right time, change it as soon as possible, then resume your original schedule. If you still have your womb, breakthrough bleeding is more likely if you forget to change your patch on time.
If you use more FemSeven than you should
If you apply too many patches, overdose is unlikely - removal of the patches is the only action required.
If you forget to use FemSeven
Do not use a double dose to make up for a forgotten dose.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, FemSeven can cause side effects; although not every body gets them.
For a list of side effects requiring discontinuation of treatment, see Section 2. “Stop using FemSeven and see a doctor immediately”
The following effects are reported more often in women using HRT compared to women not using it:
• breast cancer;
• abnormal growth or cancer of the lining of the womb (endometrial hyperplasia or cancer);
• ovarian cancer;
• blood clots in the veins of the legs or lungs (venous thromboembolism);
• heart disease;
• stroke;
• probable memory loss if HRT is started over the age of 65;
For more information about these side effects, see section 2.
The following side effects may occur very commonly (in more than 1 in 10 people):
- Application site reactions:
• Itching (purities);
• Redness (erythema);
• Eczema;
• Urticaria;
• Swilling (oedema);
• Changes in skin pigmentation
They were mostly mild skin reactions and usually disappeared 2 or 3 days after patch removal.
The following side effects may occur commonly (up to 1 in 10 people):
• Headache;
• Breast discomfort (e.g. mastalgia/ mastopathies, breast enlargement).
The following side effects may occur uncommonly (up to 1 in 100 people):
• Hair changes, sweating increased;
• Joint pain (arthralgia), leg cramps;
• Dizziness, tingling in fingers or toes (paresthesia), migraine;
• Anxiety, appetite increase, depression, difficulty sleeping (insomnia), nervousness;
• Nausea, indigestion (dyspepsia), abdominal pain, vomiting;
• Blood pressure changes;
• Chest pain;
• Vein disorders;
• Vaginal discharge, breakthrough bleeding;
• Swelling (oedema), fatigue, weight changes.
The following potential side effects may occur rarely (up to 1 people in 1 000):
• Worsening of uterine fibroids (benign growths of the womb).
The following side effects have been reported with other HRTs:
• gall bladder disease
• various skin disorders:
- discoloration of the skin especially of the face or neck known as “pregnancy patches” (cholasma);
- painful reddish nodules (erythema nodosum);
- rash with target-shaped reddening or sores (erythema multiforme);
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Keep out of the reach and sight of children.
Do not use FemSeven after the expiry date which is stated on the patch. The expiry date refers to the last day of that month.
Do not store FemSeven transdermal patches above 30°C.
Keep your patches in the sachets they come in until just before you need each one
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to
dispose of medicines no longer required. These measures will help to protect the environment.
6. CONTENT OF THE PACK AND OTHER INFORMATION
What FemSeven contains
• The active substance is: estradiol hemihydrate (2.25 milligrams).
• The other ingredients are: transparent polyethylene terephthalate foil, styrene-isoprene block copolymer and glycerine esters of completely hydrogenated resins.
The patches deliver 75 micrograms of estradiol in each 24 hour period.
What FemSeven looks like and contents of the pack
FemSeven transdermal patches are octagonal transparent, flexible, patches with rounded edges. Each patch is coated with adhesive and mounted on an oversized, removable, protective liner. The adhesive is made of a mixture of polymer and modified resin containing the active ingredients.
Each pack contains 4 or 12 patches to provide you with one month’s or 3 months treatment respectively. Marketing Authorisation Holder and Manufacturer [To be completed nationally]
This medicinal product is authorised in the Member States of the EEA under the following names:
According to the current European legislation
This leaflet was last approved in {MM/YYYY}.
[To be completed nationally]
Detailed information on this medicine is available on the web site of {MA/Agency}
8