Femseven Conti
Out of date information, search anotherNOT F7 CONTI UK_NOT F7 CONTI UK 06/09/1 Pagel
NOT F7 CONTI UK_NOT F7 CONTI UK 06/09/1 Pagel
NT0FM30500XGB05
6507399/2631
■iEUZi
PACKAGE LEAFLET: INFORMATION FOR THE USER
FemSeven® Conti
50 micrograms/7 micrograms/24 hours, transdermalpatch Estradiol hemihydrate / Levonorgestrel
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
What is in this leaflet:
1. What FemSeven® Conti is and what it is used for
2. What you need to know before you use FemSeven® Conti
3. How to use FemSeven® Conti
4. Possible side effects
5. How to store FemSeven® Conti
6. Contents of the pack and other information
1. What FemSeven® Conti is and what it is used for
FemSeven® Conti is a Hormone Replacement Therapy (HRT). It contains two types of female hormones, an oestrogen (Estradiol hemihydrate) and a progestogen (levonorgestrel).
FemSeven® Conti is used in postmenopausal women more than one year after menopause.
FemSeven® Conti is used for:
Relief of symptoms occurring after menopause
During the menopause, the amount of the oestrogen produced by a woman's body drops.
This can cause symptoms such as hot face, neck and chest ("hot fl ushes"). FemSeven® Conti is used in postmenopausal women more than one year after menopause. FemSeven® Conti alleviates these symptoms after menopause. You will only be prescribed FemSeven® Conti if your symptoms seriously hinder your daily life. Experience of treating women older than 65 years is limited.
2. What you need to know before you use FemSeven®
Medical history and regular check-ups
The use of HRT carries risks which need to be considered when deciding whether to start taking it, or whether to carry on taking it.
The experience treating women with a premature menopause (due to ovarian failure or surgery) is limited. If you have a premature menopause the risk of using HRT may be different. Please talk to your doctor.
Before you start (or restart) HRT, your doctor will ask about your own and your family's medical history. Your doctor may decide to perform a physical examination. This may include an examination of your breasts and/or an internal examination, if necessary.
Once you have started on FemSeven® Conti you should see your doctor for regular check-ups (at least once a year). At these check-ups, discuss with your doctor the benefi ts and risks of continuing with FemSeven® Conti.
Go for regular breast screening, as recommended by your doctor.
Do not use FemSeven® Conti
If any of the following applies to you. If you are not sure about any of the points below, talk to your doctor before using FemSeven® Conti.
Do not use FemSeven® Conti
• If you have or have ever had breast cancer, or if you are suspected of having it.
• If you have cancer which is sensitive to oestrogens, such as cancer of the womb lining (endometrium), or if you are suspected of having it.
• If you have any unexplained vaginal bleeding.
• If you have excessive thickening of the womb lining (endometrial hyperplasia) that is not being treated.
• If you have or have ever had a blood clot in a vein (thrombosis), such as in the legs (deep venous thrombosis) or the lungs (pulmonary embolism).
• If you have a blood clotting disorder (such as protein C, protein S, or antithrombin deficiency).
• If you have or recently have had a disease caused by blood clots in the arteries, such as a heart attack, stroke or angina.
• If you have or have ever had a liver disease and your liver function tests have not returned to normal.
• If you have a rare blood problem called "porphyria" which is passed down in families (inherited).
• If you are allergic (hypersensitive) to one of the active substances (estradiol hemihydrate and/or levonorgestrel) or any of the other ingredients of FemSeven® Conti (listed in section 6).
If any of the above conditions appear for the first time while using FemSeven® Conti, stop using it at once and consult your doctor immediately.
Warnings and precautions
Tell your doctor if you have ever had any of the following problems, before you start the treatment, as these may return or become worse during treatment with FemSeven® Conti. If so, you should see your doctor more often for check-ups:
• fibroids inside your womb;
• growth of womb lining outside your womb (endometriosis) or a history of abnormal growth of the womb lining (endometrial hyperplasia);
• increased risk of developing blood clots (see "Blood clots in a vein (thrombosis)");
• increased risk of getting an oestrogen-sensitive cancer (such as having a mother, sister or grandmother who has had breast cancer);
• high blood pressure;
• a liver disorder, such as a benign liver tumour;
• diabetes;
• gallstones;
• migraine or severe headaches;
• a disease of the immune system that affects many organs of the body (systemic lupus erythematosus, SLE);
• epilepsy;
• asthma;
• a disease affecting the eardrum and hearing (otosclerosis);
• a very high level of fat (triglycerides) in your blood;
• fluid retention due to cardiac or kidney problems.
Stop using FemSeven® Conti and see a doctor immediately: If you notice any of the following when using HRT:
• any of the conditions mentioned in the "Do not use FemSeven® Conti" section;
• yellowing of your skin or the whites of your eyes (jaundice). These may be signs of a liver disease;
• a large rise in your blood pressure (symptoms may be headache, tiredness, dizziness);
• migraine-like headaches which happen for the first time;
• if you become pregnant;
• if you notice signs of a blood clot, such as:
- painful swelling and redness of the legs;
- sudden chest pain;
- diffi culty breathing.
For more information, see "Blood clots in a vein (thrombosis)"
Note: FemSeven® Conti is not a contraceptive. If it is less than 12 months since your last menstrual period or you are under 50 years old, you may need to use additional contraception to prevent pregnancy. Speak to your doctor for advice.
FemSeven® Conti and cancer Excessive thickening of the lining of the womb (endometrial hyperplasia) and cancer of the lining of the womb (endometrial cancer).
Taking oestrogen-only HRT will increase the risk of excessive thickening of the lining of the womb (endometrial hyperplasia) and cancer of the womb lining (endometrial cancer).
The progestogen in FemSeven® Conti protects you from this extra risk.
In women who still have a womb and who are not taking HRT, on average, 5 in 1 000 will be diagnosed with endometrial cancer between the ages of 50 and 65.
For women aged 50 to 65 who still have a womb and who take oestrogen-only HRT, between 10 and 60 women in 1 000 will be diagnosed with endometrial cancer (i.e. between 5 and 55 extra cases), depending on the dose and for how long it is taken.
Irregular bleeding
You may have irregular bleeding or drops of blood (spotting) during the first 3-6 months of using FemSeven® Conti.
However, if the irregular bleeding:
• carries on for more than the first 6 months;
• starts after you have been using FemSeven® Conti for more than 6 months;
• carries on after you have stopped using FemSeven® Conti. see your doctor as soon as possible
Breast cancer:
Evidence suggests that taking combined oestrogen-progestogen and possibly also oestrogen-only HRT increases the risk of breast cancer. The extra risk depends on how long you take HRT. The additional risk becomes clear within a few years. However, it returns to normal within a few years (at most five) after stopping treatment.
Compare
Women aged 50 to 79 who are not taking HRT, on average, 9 to 14 in 1 000 will be diagnosed with breast cancer over a 5-year period. For women aged 50 to 79 who are taking oestrogen progestogen HRT over 5 years, there will be 13 to 20 cases in 1 000 users (i.e. an extra 4 to 6 cases)
Regularly check your breasts. See your doctor if you notice any changes such as:
• dimpling of the skin;
• changes in the nipple;
• any lumps you can see or feel.
Ovarian cancer:
Ovarian cancer is rare. A slightly increased risk of ovarian cancer has been reported in women taking HRT for at least 5 to 10 years.
Women aged 50 to 69 who are not taking HRT, on average about 2 women in 1 000 will be diagnosed with ovarian cancer over a 5-year period. For women who have been taking HRT for 5 years, there will be between 2 and 3 cases per 1 000 users (i.e. up to 1 extra case)
Effect of FemSeven® Conti on heart and circulation Blood clots in a vein (thrombosis)
The risk of blood clots in the veins is about 1.3 to 3- times higher in HRT users than in nonusers, especially during the fi rst year of taking it.
Blood clots can be serious, and if one travels to the lungs, it can cause chest pain, breathlessness, fainting or even death. You are more likely to get a blood clot in your veins as you get older and if any of the following applies to you. Inform your doctor if any of these situations applies to you:
• you are unable to walk for a long time because of major surgery, injury or illness (see also section 3, if you need to have surgery);
• you are seriously overweight (BMI>30 kg/m2);
• you have any blood clotting problem that needs long-term treatment with a medicine used to prevent blood clots;
• if any of your close relatives has ever had a blood clot in the leg, lung or another organ;
• you have systemic lupus erythematosus (SLE);
• you have cancer.
For signs of a blood clot, see "Stop using FemSeven® Conti and see a doctor immediately".
Compare
Looking at women in their 50s who are not taking HRT, on average, over a 5-year period, 4 to 7 in 1000 would be expected to get a blood clot in a vein.
For women in their 50s who have been taking oestrogen-progestogen HRT for over 5 years, there will be 9 to 12 cases in 1000 users (i.e. an extra 5 cases).
Heart disease (heart attack)
There is no evidence that HRT will prevent a heart attack. Women over the age of 60 years who use oestrogen-progestogen HRT are slightly more likely to develop heart disease than those not taking any HRT.
Stroke
The risk of getting stroke is about 1.5-times higher in HRT users than in non-users. The number of extra cases of stroke due to use of HRT will increase with age.
Compare
Looking at women in their 50s who are not taking HRT, on average, 8 in 1 000 would be expected to have a stroke over a 5-year period.
For women in their 50s who are taking HRT, there will be 11 cases in 1 000 users, over 5 years (i.e. an extra 3 cases). Other conditions
HRT will not prevent memory loss. There is some evidence of a higher risk of memory loss in women who start using HRT after the age of 65.
Speak to your Doctor for advice.
Other medicines and FemSeven® Conti Some medicines may interfere with the effect of FemSeven® Conti. This might lead to irregular bleeding.
This applies to the following medicines:
- Medicines for epilepsy (such as phenobarbital, phenytoin and carbamazepin);
- Medicines for tuberculosis (such as rifampicin, rifabutin);
- Medicines for HIV infection (such as nevirapine, efavirenz, ritonavir and nelfi navir);
- Herbal remedies containing St John's Wort (Hypericum perforatum).
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines Laboratory tests
If you need a blood test, tell your doctor or the laboratory staff that you are using FemSeven® Conti, because this
medicine can affect the results of some tests. Pregnancy and breast-feeding
FemSeven® Conti is for use in postmenopausal women only. If you become pregnant, stop using FemSeven® Conti and contact your doctor.
3. How to use FemSeven® Conti
Dosage
Always use this medicine exactly as your doctor or pharmacist has told you. Check with your doctor or pharmacist if you are not sure.
• FemSeven® Conti has to be applied once a week, i.e. each patch is replaced every 7 days.
FemSeven® Conti is a continuous combined hormone replacement therapy (HRT) treatment without a treatment-off phase: as one patch is removed, the next is applied immediately
Forgetting to change a patch on schedule may increase the likelihood of break-through bleeding or spotting.
• If you are not taking HRT or you are transferring from another continuous combined HRT product, treatment with FemSeven® Conti may be started on any convenient day.
• If you are transferring from sequential HRT regimens, treatment should start right after your withdrawal bleeding has ended.
Your doctor will aim to prescribe the lowest dose to treat your symptoms for as short a time as necessary.
Speak to your doctor if you think this dose is too strong or not strong enough.
Method of administration
• FemSeven® Conti should be applied on your skin (transdermal use). Wash and clean the area thoroughly and dry the skin before application, if possible apply to skin that is free from hair.
• FemSeven® Conti should be applied to clean, dry, healthy skin (which is neither irritated nor grazed). Do not apply to skin that has been recently treated with cosmetic creams or sun protection products. Avoid using bath oils in your bath or shower gels containing moisturising or oily ingredients, as this may affect patch adhesion anywhere on the body.
• FemSeven® Conti should be applied to an area of skin without major skin folds, i.e. the buttocks or hips, and not subject to chafing by clothing (avoid the waist and avoid wearing tight clothing that could loosen the transdermal patch). Do not try to check if it has stuck by attempting to lift the edge as this may make it come loose.
• Wait at least one hour after patch application before you try any strenuous activity or exercise that will make you perspire (sweat) a lot as this can affect patch adhesion.
• Also, wait an hour after patch application before wet activity. This includes bathing, showering, swimming or use of a steam room.
• Other factors that may cause poor adhesion are:
- Excessive perspiration, hot flushes, or if you have a naturally oily skin.
- Hot and/or humid weather conditions.
• FemSeven® Conti must not be applied either on or near the breasts. It is advisable to avoid applying the patch to the same site twice running. At least one week should be allowed to elapse between applications to the same site.
If you need to have surgery
If you are going to have surgery, tell the surgeon that you are using FemSeven® Conti . You may need to stop using FemSeven® Conti about 4 to 6 weeks before the operation to reduce the risk of a blood clot (see section 2, Blood clots in vein). Ask your doctor when you can start taking FemSeven® Conti again.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
The following diseases are reported more often in women using HRT compared to women not using HRT:
• breast cancer;
• abnormal growth or cancer of the lining of the womb (endometrial hyperplasia or cancer);
• ovarian cancer;
• blood clots in the veins of the legs or lungs (venous thromboembolism);
• heart disease;
• stroke;
• probable memory loss if HRT is started over the age of 65. For more information about these side effects, see section 2.
Like all medicines, FemSeven® Conti can cause side effects, although not everybody gets them.
Most of the effects observed with FemSeven® Conti are weak to moderate and do not require the treatment to be stopped.
Should the following persist, ask your doctor's advice, who may adapt the treatment: hot flushes, headaches, inconvenient vaginal dryness, nausea, vomiting, abdominal pain, tightness in the breasts, eye irritation from contact lenses, irritability, heavy legs and weight gain.
In the case of heavy or irregular gynaecological bleeding, consult your doctor.
The most frequent side effects may occur very commonly (in more than 1 in 10 people):
• skin irritation at the site of application (disappeared 2 or 3 days after patch removal);
• breast tenderness;
• bleeding or spotting.
The following side effects may occur commonly (up to 1 in 10 people):
• breast pain (mastodynia);
• headache;
• indigestion (dyspepsia).
The following side effects may occur uncommonly (up to 1 in 100 people):
• fluid retention, swelling (oedema),
• weight increase/loss;
• fatigue;
• leg cramps;
• dizziness
• migraine;
• bloating;
• abdominal cramps;
• feeling sick (nausea);
• hypertension;
• excessive thickening of the womb lining (endometrial hyperplasia);
• benign breast tissue changes;
• depression.
The following potential side effects may occur rarely (up to 1 in 1,000 people):
• presence of gallstones in the gallbladder (cholelithiasis) ;
• yellowing of your skin or the whites of your eyes (cholestatic jaundice);
• increase in size of fibroids inside your womb (uterine fibrosis).
The following side effects have been reported with other HRTs:
• gall bladder disease;
• various skin disorders:
- discoloration of the skin especially of the face or neck known as "pregnancy patches" (chloasma);
- painful reddish nodules (erythema nodosum);
- rash with target-shaped reddening or sores (erythema multiforme);
- vascular purpura (occasional reddening of the skin).
If you get any side effects, talk to your doctor or pharmacist. This includes any side effects not listed in this leaflet.
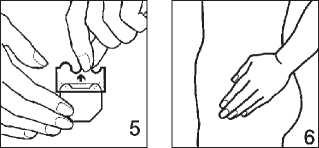
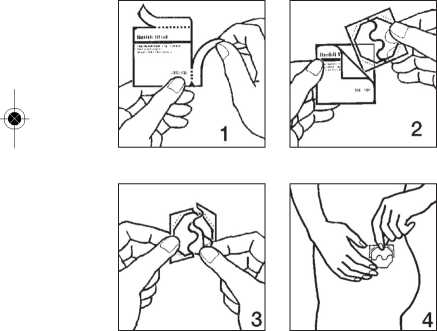
Putting on the patch:
1. Remove the patch from its pouch as shown in pictures 1 and 2.
2. Peel off half the protective liner at the S-shaped notch and apply the patch to the skin as in pictures 3 and 4. Avoid touching the adhesive side of the patch with your fingers as this may prevent it sticking properly later on.
3. Remove the other half of the protective liner then press the patch against your skin with the palm of your hand for at least 30 seconds shown in pictures 5 and 6. The warmth of your body will make the patch stick better.
• It is possible to take a shower or have a bath without removing the transdermal patch. In the event that the transdermal patch should become detached prematurely, i.e. before the seventh day (due to vigorous physical activity, excessive sweating, abnormal chafing of clothing), a new patch should be applied (to aid compliance it is recommended that the patient then continues to change the patch on the original scheduled day).
• Once applied, the transdermal patch has to be covered by clothes to avoid direct exposure to sunlight.
• Removal of the transdermal patch should be carried out slowly to avoid irritating the skin. In the event of some of the adhesive remaining on the skin, this can usually be removed by gently rubbing with a cream or an oily lotion.
• After use, FemSeven® Conti is to be folded in two (with the adhesive surface to the inside) and disposed of.
If you use more FemSeven® Conti than you should
Overdose is unlikely but it can cause the following:
• breast tenderness;
• swelling of the abdomen/pelvis;
• anxiety;
• irritability;
• feeling sick (nausea);
• vomiting.
These symptoms will disappear gradually on removal of the patches.
Should the signs persist, ask your doctor's advice.
If you forget to change your patch of FemSeven® Conti
Change your patch as soon as possible, then resume your original schedule. Breakthrough bleeding is more likely if you forget to change your patch on time.
Do not take a double dose to compensate for the patch you forget to change.
If you stop using FemSeven® Conti
The premenopausal signs linked to a lack of oestrogen (such as hot face, neck and chest) may reappear.
5. How to store FemSeven® Conti
Keep this medicine out of the sight and reach of children. Do not use this medicine after the expiry date which is stated on the pack. The expiry date refers to the last day of that month.
Do not store FemSeven® Conti above 30°C.
Keep your patches in the sachets they come in until just before you need each one.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information
What FemSeven® Conti contains
• The active substances are: estradiol hemihydrate and levonorgestrel. Each patch contains 1.5 mg of estradiol hemihydrate and 0.525 mg of levonorgestrel in a patch size of 15 cm2, releasing 50 micrograms of estradiol and 7 micrograms of levonorgestrel per 24 hours.
• The other ingredients are:
Backing layer: Polyethylene terephthalate (PET) foil. Adhesive matrix: Styrene-isoprene-styrene block copolymer, glycerine esters of completely hydrogenated resins. Protective liner:SiIiconised polyethylene terephthalate (PET) foil.
What FemSeven® Conti looks like and contents of the pack
FemSeven® Conti is a transdermal patch contained in a sachet. Each pack contains 4 or 12 sachets.
Marketing Authorisation Holder and Manufacturer FemSeven Conti is manufactured by Laboratoire Theramex, 6 Avenue Albert II, 98000 Monaco for Teva UK Limited, Eastbourne, BN22 9AG.
This medicinal product is authorised in the Member States of the EEA under the following names: Fem7 Evo/Fem7 Plus/FemSeven Conti This leaflet was last revised in 07/2012.
Detailed information on this medicine is available on the web site of the Medicines and Healthcare products Regulatory Agency (MHRA).
NT0FM30500XGB05
6507399/2631
-fo-