Femseven Sequi
Pagel
Pagel
NT0FM20500XGB07 6507396 / 2630
PATIENT INFORMATION LEAFLET
FemSeven® Sequi 50 micrograms/10 micrograms/24 hours transdermal patch
(Estradiol/levonorgestrel)
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What FEMSEVEN SEQUI®, PATCH is and what it is used for
2. Before you use FEMSEVEN SEQUI ®, PATCH
3. How to use FEMSEVEN SEQUI ®, PATCH
4. Possible side effects
5. How to store FEMSEVEN SEQUI®, PATCH
6. Further information
1. WHAT FEMSEVEN SEQUI®, PATCH IS AND WHAT IT IS USED FOR
FEMSEVEN SEQUI® is a sequential combined Hormone Replacement Therapy (HRT). It contains an oestrogen (estradiol) and a progestogen (levonorgestrel). FEMSEVEN SEQUI® is suitable for postmenopausal women.
During the menopause, the amount of oestrogen produced by a woman's body drops. For some women this can cause symptoms such as hot face, neck and chest ("hot flushes"). FEMSEVEN SEQUI® alleviates these symptoms after menopause.
The experience treating women above the age of 65 is limited.
2. BEFORE YOU USE FEMSEVEN SEQUI®, PATCH
You should fully inform your doctor about your personal medical history and that of your immediate family, before starting with hormone replacement therapy (HRT)
Medical examinations and tests
Your doctor willask you to undergo a medical examination before beginning treatment then regularly during the treatment (at least once a year) to check whether your body is tolerating the medication.
Your breasts will be regularly examined by your doctor, especially if you have any lumps (cysts or nodules) or if anybody in your family has already had breast cancer. Your doctor may ask you to have a mammography.
From time to time, at least once a year, the risks and benefits of HRT treatment should be carefully reassessed to determine if the treatment should be continued.
Do not use FEMSEVEN SEQUI®, PATCH
- if you are allergic (hypersensitive) to one of the active substances (Estradiol or Levonorgestrel) or any of the other ingredients of FEMSEVEN SEQUI® (see also section
6).
- If you have, have had or your doctor thinks you might have breast cancer.
- if you have previously had a blockage of one of your veins in your leg or lungs (deep venous thrombosis or lung embolism), or if you currently have such a blockage
- if you have thrombophilic disorders (e.g. protein C, protein S, or anthithrombin deficiency)
- if you currently have or have recently had a blockage of an artery, for example, angina pectoris (heart cramp due to an oxygen deficiency) a heart attack or stroke
- If you have, or your doctor thinks you might have, breast, uterine or any other cancer requiring a hormone to develop (oestrogen-dependent cancer).
- If there is excessive proliferation of cells in the inner lining (endometrial hyperplasia) that has not been treated yet.
- If you experience unexplained vaginal bleeds.
- If you currently have or have previously had a liver disorder. You must not use FEMSEVEN SEQUI® for as long as the liver function has not returned to normal - If you have a (herediatary) disorder in the composition of the blood pigment (porphyria).
Take special care with FEMSEVEN SEQUI®, PATCH
Tell your doctor if you have or have previously had any of the following disorders and/or if these conditions have been aggravated during pregnancy or previous hormone therapy, before you start using FEMSEVEN SEQUI®.
- If you have high blood pressure,
- If you have an increased risk of developing blood clots (see “HRT and effects on heart or blood circulation”),
- If you have a high blood sugar level (diabetes) with or without vascular disorders
- If you have a disease leading to the presence of uterine mucosa outside your uterus causing pain and bleeding (endometriosis),
- If you have a benign tumour in your uterus (uterine fibroma),
- If you have a history of excessive proliferation of cells in the inner lining (endometrial hyperplasia),
- If you have an increased risk of developing tumours related to the levels of oestrogens in the blood (such as having a close relative with breast cancer)
- If you have liver or gallbladder disease (hepatic adenoma, gallstones),
- If you suffer from epilepsy,
- If you have severe headaches or migraines,
- If you have asthma,
- If you have a serious immune disease that affects your skin in particular (lupus),
- If you have a disease causing loss of hearing (otosclerosis).
Stop using FEMSEVEN SEQUI® immediately
If you experience any of the disorders mentioned under “Do not use FEMSEVEN SEQUI®, PATCH”, or if any of the following situations occurs:
- you experience a yellowing of the skin (jaundice) or your liver function deteriorates;
- your blood pressure suddenly becomes much higher;
- you get a migraine-like headache for the first time;
- you become pregnant.
Note: FEMSEVEN SEQUI® is not a transdermal contraceptive and does not prevent you from becoming pregnant.
You must also inform your doctor if:
- You are to undergo surgery.
- You have to remain immobilised for a long period.
- You have contracted another disease.
What risks are associated with the use of FEMSEVEN SEQUI® PATCH?
HRT and effects on heart or blood circulation Blood clots (thrombosis):
Immediately inform your doctor if you have painful swelling of one of your legs, sudden pain in the chest, or shortness of breath while using FEMSEVEN SEQUI® PATCH. This could be a sign of venous thrombosis or a lung embolism while, in which case you must stop using FEMSEVEN SEQUI® PATCH immediately.
HRT increases the risk of VTE 1.3-3 fold, especially during the first year of taking it.
Compare
Looking at women in their 50s who are not taking HRT, on average, over a 5-year period, 4 in 1000 would be expected to get a blood clot.
For women in their 50s who are taking HRT over 5 years, the number of extra cases will be 5 in 1000 users.
The risk of venous thrombosis is greater:
• If you use oestrogens;
• With older age;
• If you have a cancer;
• During pregnancy and postpartum period;
• If there is venous thrombosis in your immediate family;
• If you are severely overweight
• If you have systemic lupus erythematosus (a disorder of the immune system);
• If you have any blood clotting problem that needs longterm treatment with a medicine used to prevent blood clots
• If you are immobilised for long periods (e.g. when you must have bed rest), have an accident or a major surgery. In these circumstances it might be necessary for you to stop using FEMSEVEN SEQUI® PATCH temporarily. You may need to stop as early 4-6 weeks before planned surgery.
It is unclear whether having varicose veins leads to an increased risk of venous thrombosis.
If any of these situations apply to you, please inform your doctor. If you are using an anticoagulant, the risks and benefits of using HRT have to be carefully evaluated. Disorders of the coronary arteries:
Stop using FEMSEVEN SEQUI® PATCH and contact your doctor immediately, if you get a pain in your chest that spreads to your arm or neck. The pain may be a sign of a heart disease.
There is no evidence that HRT will help to prevent heart disease. Women taking oestrogen-progestogen HRT are slightly more likely to get heart disease than those not taking any HRT. As the risk of CAD strongly depends on age, the number of extra cases of CAD due to oestrogen-progestogen use is very low in healthy women close to menopause, but will rise with more advanced age.
Risk of stroke:
Stop using FEMSEVEN SEQUI® PATCH and contact your doctor immediately if you get: unexplained migraine-like headaches, with or without disturbed vision. Such headaches can be an early sign of a stroke.
Combined oestrogen-progestogen and oestrogen-only HRT increase the risk of stroke up to 1.5-fold. The risk of users compared to non-users does not change with age or time since menopause. However, as the risk of stroke is strongly age-dependent, the overall risk of stroke in women who use HRT will increase with age.
Compare
Looking at women in their 50s who are not taking HRT, on average, over a 5-year period, 8 in 1000 would be expected to have a stroke.
For women in their 50s who are taking HRT over 5 years, the number of extra cases will be 3 in 1000 users.
HRT and risks of developing cancer
Overgrowth of the lining of the womb (endometrial
hyperplasia) and cancer of the womb lining (endometrial
cancer).
Long-term use of estrogen increases the risk of overgrowth of the lining of the womb (endometrial hyperplasis) and the risk of endometrial cancer in women with an uterus. To reduce this risk it is necessary to take the estrogens in combination with progestogen tablets for at least 12 days of each month.
During the first months of treatment irregular bleeding may occur. Contact your doctor if - such bleeding carries on for more that the first few months of treatment
- starts after you have been on FEMSEVEN SEQUI® PATCH for a while - carries on even after you have stopped using FEMSEVEN SEQUI® PATCH
Your doctor will examine what the cause is which may include a biopsy of the womb lining in order to find out whether you have cancer of the womb lining.
Compare
Looking at women who still have a womb and who are not taking HRT, on average 5 in 1000 will be diagnosed with endometrial cancer.
For women who take oestrogen-only HRT, the number of extra-cases will be between 5 and 55 in 1000 users between the ages of 50 and 65 depending on the dose and for how long it is taken.
The addition of a progestogen to oestrogen-only HRT substantially reduces the risk of endometrial cancer. Breast cancer:
Evidence suggests that taking combined oestrogen-progestogen and possibly also oestrogen-only HRT increases the risk of breast cancer. This depends on how long you take HRT, and the extra risk is visible after about 3 years. However, it returns to normal within a few (at most five) years after stopping.
Contact your doctor if you notice changes to your breasts, such as: dimpling of the breast, skin changes in the nipple, lumps that you can see or feel.
Your doctor may advice you to undergo a check up including mammography.
Compare
Looking at women aged 50 to 65 who are not taking HRT, on average 9-12 in 1000 will be diagnosed with breast cancer over a 5 year period.
For women aged 50-65 who are taking oestrogen plus progestogen HRT over 5 years the number of extra-cases will be 6 in 1000 users.
Looking at women aged 50 to 79 who are not taking HRT, on average 14 in 1000 will be diagnosed with breast cancer over a 5 year period.
For women aged 50-79 who are taking oestrogen plus progestogen HRT over 5 years the number of extra cases will be 4 in 1000 users.
Ovarian cancer:
Ovarian cancer is much rarer than breast cancer. Longterm (at least 5-10 years) use of oestrogen-only HRT products is thought to carry a slightly increased risk of ovarian cancer. Some studies suggest that the long-term use of combined HRTs may carry a similar, or slightly smaller, risk. For women who are taking HRT over 5 years there will be one extra case per 2500 users.
HRT and other disorders:
• If you suffer from cardiac or kidney dysfunction you will have to be monitored while using FEMSEVEN SEQUI® PATCH • If you have very high triglyceride levels in your blood (hypertriglyceridaemia) you will have to be monitored when using FEMSEVEN SEQUI® PATCH.
HRT will not improve thought processes. There are hints of an increased risk of probable dementia in women who start using HRT after the age of 65.
Consult your doctor if one of the above-mentioned warnings should apply to you, or if it has previously applied to you.
Taking or using other medicines
Some medications can alter the effectiveness of FEMSEVEN SEQUI® in particular if you are taking:
- a medicine to treat epilepsy (carbamazepin, phenobarbital, phenytoin),
- a medicine to treat tuberculosis or other infectious diseases (rifampicin, rifabutin),
- a medicine to treat AIDS (ritonavir, nelfinavir, nevirapine, efavirenz),

- a medicine containing St. John's Wort (Hypericum perforatum).
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Pregnancy and breast-feeding
You should not use FEMSEVEN SEQUI® if you are
pregnant or breastfeeding.
If you discover that you are pregnant while using this medication, stop the treatment and discuss the situation with your doctor.
Ask your doctor or pharmacist for advice before taking any medicine.
Driving and using machines
No specific side effect is expected.
3. HOW TO USE FEMSEVEN SEQUI®, PATCH
Always use FEMSEVEN SEQUI® exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
You should apply the patch once a week i.e. replace it every 7 days without a break in treatment: as soon as you remove one patch, you should apply another one to your skin.
There are 2 types of patches in the box, depending on when you apply one:
- Phase 1 patches (1st and 2nd weeks of your cycle): they contain only one active substance (Estradiol).
- Phase 2 patches (3rd and 4th weeks of your cycle): they contain both active substances (Estradioland Levonorgestrel).
If you are not taking HRT or you switch from a continuous combined HRT product, treatment may be started on any convenient day.
If you are transferring from a sequential HRT regimen, treatment should begin the day following completion of the prior regimen.
Frequency of application:
1. Apply a Phase 1 patch once a week for the first 2 weeks of your cycle.
2. Remove the Phase 1 patch and apply a Phase 2 patch in its place, once a week for the following 2 weeks.
You must apply the patches in the correct order.
Bleeding similar to periods usually occurs at the end of the use of the Phase 2 patches. The bleeding is light and lasts for 4 to 5 days on average.
If the bleeding is heavy or irregular, consult your doctor.
Method of administration
This medicine should be applied to the skin.
How to apply a patch?
Each sachet contains one patch.
FEMSEVEN SEQUI® must be applied immediately after being removed from its sachet.
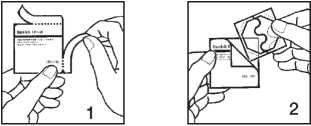
1. To open the sachet, tear the two edges in the direction of the arrow.
2. Remove the patch from the sachet. A patch has two sections: the patch itself, which must be applied to the skin, and the protective liner.
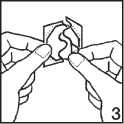
3. Remove one-half of the liner, starting at the S-shaped notch and taking care not to touch the adhesive side of the patch with your fingers. If you do, it may not adhere correctly and you may alter the active ingredients.
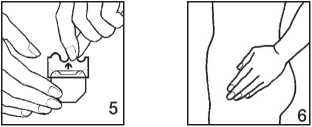
4. Apply the patch immediately, using the palm of your hand, to a dry, clean area of skin. It should not be red or covered with cream or lotion. The location should not have any significant wrinkles and it should not be rubbed by clothing (avoid placing at the waist and do not wear very tight clothing). The patch may be applied, for example, to the buttocks, thighs, abdomen etc.
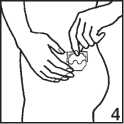
5. Remove the other part of the plastic liner and press down on the corresponding area of the patch.
6. Keep the palm of your hand on the patch for at least 30 seconds to ensure that it is adhering correctly, especially round the edges. The pressure and heat of the hand are essential to ensure maximum adhesion.
How to remove the patch?
To remove a FEMSEVEN SEQUI®, simply free up one edge and pull it off gently so as not to irritate the skin. If any of the adhesive remains on the skin, you can remove it by rubbing the skin gently with a cream or an oily lotion. After use, the patch still contains active substances, but in too small a quantity for it to remain effective. Fold the patch in half (adhesive to adhesive) before discarding it. Precautions for use
Do not apply a FEMSEVEN SEQUI® PATCH:
- to your breasts.
- Twice in the same place: leave at least 1 week between 2 applications in the same place.
During the course of treatment:
- You must not expose the patch directly to the sun once you have applied it to your skin.
- You can shower or take a bath with the patch on your skin.
- If the patch becomes unstuck before the end of its use i.e. before the 7th day (for example if you have undertaken intense physical effort or perspire abundantly or the skin is rubbed by clothes), use a new patch (from the same phase) and remove it at the initially scheduled date.
Duration of treatment
Your doctor will decide on the duration of treatment. Contact your doctor if you want to stop the treatment.
If you take more FEMSEVEN SEQUI® than you should
Overdose is unlikely but it can cause the following:
- pain in the breasts,
- bloating in the abdomen, flatulence, nausea and vomiting,
- irritability, anxiety,
- vaginal bleeding.
No specific treatment is required. The signs will disappear when the patch is removed.
If you forget to take FEMSEVEN SEQUI®, PATCH
- If you have forgotten to change the patch on the scheduled day, replace it immediately then follow the treatment normally, changing the patch again on the initially scheduled day.
- Do not use 2 patches at the same time to make up for the single dose that you have forgotten.
- If you have not used FEMSEVEN SEQUI ® for several successive days, you may get stop bleeding. If you are in doubt, consult your doctor.
If you stop using FEMSEVEN SEQUI®, PATCH The postmenopausal signs linked to a lack of oestrogen may reappear.
If you have any further questions, ask your doctor or your pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, FEMSEVEN SEQUI® can cause side effects, although not everybody gets them.
The following side effects may occur the most frequently (in more than 1 in 10 patients treated).
- Skin reaction (itching, irritation, redness) in the area in which the patch is applied. These signs are not serious and usually disappear 2 or 3 days after removing the patch. If they persist, place the patch in a different area. The following side effects may occur frequently (in 1 to 10 people in 100):
- Tightness or pain in the breasts.
- Headaches.
- Nausea, vomiting.
- Irregular bleeding, spotting
- Increase or decrease in sexual desire.
The following potential side effects are less frequent (in 1 to 10 people in 1,000):
- Benign breast tumour.
- Painful periods.
- Abnormal development of the lining of the uterus (endometrial hyperplasia).
- High blood pressure.
- Bloating or pain in the abdomen.
- Fatigue.
- Weight gain or loss.
- Migraine.
- Vertigo.
- Cramp in the legs.
- Swelling (water retention, oedema).
The following side effects may occur but are rare (1 to 10 people in 10,000):
- Yellowing of the skin and whites of the eye (jaundice).
- Benign tumour in the uterus (uterine fibroma).
- Gall stones
- Depression.
The following side effects can also occur:
- Breast cancer or uterine (endometrial) cancer.
- Formation of a blood clot in a vein in the leg (phlebitis) or lungs (pulmonary embolus).
- Formation of a blood clot in brain (stroke) or heart (heart attack).
- Gallbladder problems.
- Mental disorders (dementia).
- Disorders of the skin, or subcutaneous disorders such as:
• Formation of brown spots on the face after exposure to sunlight, also referred to as a mask of pregnancy (chloasma).
• Skin disease with spots in the form of red marks and blisters filled with liquid (erythema multiforme).
- Skin disease with the formation of lumps under the skin that are red and painful (erythema nodosum).
• Minor skin bleeds (Henoch-Schonlein purpura).
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
5. HOW TO STORE FEMSEVEN SEQUI®, PATCH
Keep out of the reach and sight of children.
Do not use a FEMSEVEN SEQUI® PATCH after the expiry date which is stated on the box. The expiry date refers to the last day of that month.
Store below 30°C.
Do not use a FEMSEVEN SEQUI® PATCH if you notice any visible signs of deterioration of the patch.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. FURTHER INFORMATION
What FEMSEVEN SEQUI®, PATCH contains
• The active substances are: - Phase 1 patch: Estradiol hemihydrate (1.50 mg) for 15 cm2 patch providing 50 micrograms Estradiol every 24 hours.
- Phase 2 patch: Estradiol hemihydrate (1.50 mg) and Levonorgestrel (1.50 mg) for 15 cm2 patch providing 50 micrograms Estradiol and 10 micrograms Levonorgestrel every 24 hours.
• The other ingredients are:
- Liner: transparent polyethylene terephtalate (PET) film.
- Adhesive component: styrene-isoprene-styrene copolymer beads, glycerin esters of totally hydrogenated resin acids.
- Backing: silicon-coated transparent polyethylene terephtalate (PET) film.
What FEMSEVEN SEQUI®, PATCH looks like and contents of the pack
The product is a transparent, flexible, octagonal patch with rounded edges. The backing is larger and detachable. Each box contains 4 patches (2 Phase 1 patches and 2 Phase 2 patches) or 12 patches (6 Phase 1 patches and 6 Phase 2 patches), each individually wrapped.
Not all box sizes may be marketed.
Marketing Authorisation Holder and Manufacturer Marketing Authorisation Holder:
Teva UK Limited, Eastbourne, BN22 9AG.
Manufacturer :
LABORATOIRE THERAMEX 6, avenue Albert II, 98000 Monaco, MONACO
This medicinal product is authorised in the Member
States of the EEA under the following names:
Germany, Luxemburg:
FEM7 COMBI® 50 microgams/10 micrograms/24 hours, transdermal patch Austria, Finland:
FEMSEVENCOMBI® 50 microgams/10 micrograms/24 hours,
dispositif transdermique
Belgium:
FEMINOVA PLUS® 50 microgams/10 micrograms/24 hours,
dispositif transdermique
Spain:
COMBIFEM® 50 microgams/10 micrograms/24 hours,
dispositif transdermique
France:
FEMSEPTCOMBI® 50 microgams/10 micrograms/24 hours, dispositif transdermique United Kingdom:
FEMSEVEN SEQUI® 50 microgams/10 micrograms/24 hours,
dispositif transdermique
Italy:
COMBISEVEN® 50 microgams/10 micrograms/24 hours,
dispositif transdermique
Netherlands:
FEM7 SEQUI® 50 microgams/10 micrograms/24 hours,
dispositif transdermique
Portugal:
FEMSETE COMBI® 50 microgams/10 micrograms/24 hours, dispositif transdermique
This leaflet was last approved in 12/2012.
NT0FM20500XGB07 6507396 / 2630