Fenylat 75 Micrograms/Hour Transdermal Patch
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Fenylat 75 micrograms/hour transdermal patch
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Fenylat 75 micrograms/hour: 1 transdermal patch contains 15.3 mg fentanyl in a patch size of 25.5 cm and releases 75 micrograms fentanyl per hour.
Excipient with known effect: soya oil
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Transdermal patch
Fenylat is an opaque, colourless, rectangular shaped patch with round corners and imprint on the backing foil:
“Fentanyl 75pg/h” for Fenylat 75 micrograms/hour
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Adults
Long -term management of severe chronic pain which can be managed adequately only with opioid analgesics.
Children
Long-term management of severe chronic pain which can be managed adequately only with opioid analgesics in opioid-tolerant children receiving opioid therapy from 2 years of age (see section 4.2).
4.2 Posology and method of administration
The appropriate dose of Fenylat and the need for continued treatment should be assessed at regular intervals.
Fenylat transdermal patches release fentanyl for a period of 72 hours (corresponding to a release rate of 12.5, 25, 50, 75 and 100 micrograms/hour with a releasing surface of 4.25, 8.5, 17, 25.5 and 34 cm respectively).
Posology:
Adults
Initial dose selection
For the determination of the appropriate dose for the treatment of chronic pain, the analgesic - especially opioid analgesics - which has been administered so far must be taken into consideration. The dose, effectiveness and possible tolerance development are all reviewed for the calculation of the quantity of fentanyl required. Other factors to be considered are the current general condition and medical status of the patient, including body size, age, and extent of debilitation as well as degree of opioid tolerance.
Initial adjustment in opioid-naive patients
Clinical experience with Fenylat is limited in opioid-naive patients. In the circumstances in which therapy with Fenylat is considered appropriate in opioid-naive patients, it is recommended for these patients to be titrated with low doses of short acting analgesics. Only then the patients can be transfered to fentanyl transdermal patches. Patches with a release rate of 12.5 micrograms/h are available and should be used for initial adjustment. After an initial assessment period, patients can be transfered to fentanyl transdermal patches with a release rate of 25 micrograms/h. The dose may subsequently be titrated upwards or downwards, if required, in increments of 12 or 25 micrograms/h to achieve the lowest appropriate dose of Fenylat depending on the response and supplementary analgesic requirements (see also section 4.4).
In elderly or weak opioid-naive patients, who are particularly susceptible to opioid treatments, it is not recommended to initiate an opioid treatment with Fenylat. In these cases, it would be preferable to initiate a treatment with low doses of immediate release morphine and to prescribe Fenylat after determination of the optimal dosage.
Changes from other intensively active opioids
When changing over from oral or parenteral opioids to Fenylat, the initial dosage should be calculated as follows:
1. The quantity of analgesics required over the last 24 hours should be determined and converted to the equianalgesic oral morphine dose.
2. The corresponding fentanyl dosage should be determined as follows:
a) using Table 1 for patients who have a need for opioid rotation (conversion ratio from oral morphine to transdermal fentanyl equals 150:1)
b) using Table 2 for patients on stable and well tolerated opioid therapy
(conversion ratio from oral morphine to transdermal fentanyl equals 100:1)
Table 1: Recommended initial dose of Fenylat based on daily oral morphine dose (for patients who have a need for opioid rotation due to experienced adverse drug reactions) / conversion ratio 150:1
|
Oral morphine dose (mg/day) |
Fenylat (micrograms/h) |
|
< 90 |
12 |
|
90 - 134 (for adults) |
25 |
|
135-224 |
50 |
|
225-314 |
75 |
|
315-404 |
100 |
|
405-494 |
125 |
|
495-584 |
150 |
|
585-674 |
175 |
|
675-764 |
200 |
|
765-854 |
225 |
|
855-944 |
250 |
|
945-1034 |
275 |
|
1035-1124 |
300 |
Table 2: Recommended initial dose of Fenylat based on daily oral morphine dose
(for patients on stable and well tolerated opioid therapy) / conversion ratio
100:1
|
Oral morphine dose (mg/day) |
Fenylat (micrograms/h) |
|
< 60 |
12 |
|
60-89 |
25 |
|
90-149 |
50 |
|
150-209 |
75 |
|
210-269 |
100 |
|
270-329 |
125 |
|
330-389 |
150 |
|
390-449 |
175 |
|
450-509 |
200 |
|
510-569 |
225 |
|
570-629 |
250 |
|
630-689 |
275 |
|
690-749 |
300 |
In the cases of initial adjustment and changing from other analgesics, the maximum analgesic effect cannot be evaluated until about 24 hours have elapsed, due to gradual increase in the serum fentanyl concentrations up to this time.
Previous analgesic therapy should be phased out gradually from the time of the first patch application until analgesic efficacy with Fenylat is attained.
By combining several transdermal patches, a fentanyl release rate of more than 100 micrograms/h can be achieved.
Dose titration and maintenance therapy
For a dose titration Fenylat 12 micrograms/h with the lowest dosage is available.
Fenylat patches should be replaced every 72 hours. The dose should be titrated individually until analgesic efficacy is attained. If analgesia is insufficient at the end of the initial application period, the dose may be increased after three days. Subsequently, dose adjustments may be made every three days. Following initiation of therapy, some patients may require replacement after 48 hours instead of 72 hours should analgesia be insufficient on day 3. Replacement earlier than at 72 hours may result in an increase of fentanyl serum concentration (see section 5.2). Dose adjustment, when necessary, should normally increase from 12 micrograms/h to 25 micrograms/h while the supplementary analgesic requirements (45/90 mg oral morphine/day ~ Fenylat 12/25 micrograms/h) and pain status of the patient should be taken into account. More than one Fenylat patch may be used to achieve the desired dose. Patients may require periodic supplementary doses of a short-acting analgesic for 'breakthrough' pain. Additional or alternative methods of analgesia should be considered when the Fenylat dose exceeds 300 micrograms/h.
Changing or ending therapy
If discontinuation of Fenylat is necessary, any replacement with other opioids should be gradual, starting at a low dose and increasing slowly. This is because fentanyl levels fall gradually after the patch is removed. It takes at least 17 hours for the fentanyl serum concentration to decrease by 50 %. As a general rule, the discontinuation of opioid analgesia should be gradual, in order to prevent withdrawal symptoms.
Opioid withdrawal symptoms are possible in some patients after conversion or dose adjustment (see section 4.8). Tables 1 and 2 should not be used to calculate the switch from Fenylat to other therapies in order to avoid an overestimation of the new analgesic dose and potential overdose.
Elderly patients
Elderly should be observed carefully and the dose reduced if necessary (see sections 4.4 and 5.2).
Liver and renal impairments
Patients with hepatic or renal impairment should be observed carefully and the dose reduced if necessary (see section 4.4).
Paediatric population
Children aged 16 years and above:
Follow adult dosage.
Children aged 2 to 16 years old:
Fenylat should be administered only to opioid-tolerant paediatric patients (ages 2 to 16 years) who are already receiving at least 30 mg oral morphine equivalent per day.
Initiation / conversion of treatment to Fenylat
To convert from oral or parenteral opioids to Fenylat, the initial dose should be determined on the previous needs and the pain status (see table 3).
Table 3: Recommended Fenylat dose based upon daily oral morphine dose1
|
Oral 24-h morphine dose (mg/day) |
Fenylat (micrograms/h) |
|
2 For paediatric patients | |
|
30 - 44 |
12 |
|
45 - 134 |
25 |
1 In clinical trials these ranges of daily oral morphine doses were used as a basis for conversion to Fenylat
2 Conversions to Fenylat for doses greater than 25 micrograms/h are similar for adult and paediatric patients.
For children who receive more than 90 mg oral morphine equivalent a day, only limited information is currently available from clinical trials with transdermal fentanyl. In these paediatric studies, the required strength of fentanyl transdermal patch was calculated conservatively: 30 mg to 44 mg oral morphine per day or its equivalent opioid dose was replaced by one <invented name > 12 micrograms/h patch. It should be noted that this conversion schedule for children only applies to the switch from oral morphine (or its equivalent) to Fenylat. The conversion schedule should not be used to convert from Fenylat into other analgesics, as overdosing could then occur.
The analgesic effect of the first dose of Fenylat will not be optimal within the first 24 hours. Therefore, during the first 12 hours after switching to Fenylat, the patient should be given regular doses of the previous analgesics. In the following 12 hours, these previous analgesics should be provided based on clinical need.
Since peak fentanyl levels occur after 12 to 24 hours of treatment, monitoring of the patient for adverse events, e.g. hypoventilation, is recommended for at least 48 hours after initiation of fentanyl therapy or up-titration of the dose (see also section 4.4).
Dose titration and maintenance
If the analgesic effect of Fenylat is insufficient, supplementary morphine or another short-duration opioid should be administered in paediatric patients. Depending on the additional analgesic needs and the pain status of the child, it may be decided to increase the dose. Dose adjustments should be done in 12 micrograms fentanyl/hour steps.
Method of administration:
For transdermal use.
Immediately after the transdermal patch has been removed from the package and the release film as well as both sections of the backing film have been detached, the transdermal patch is attached to a hairless part of the skin or if this is not possible, hair at the application site should be clipped (not shaved) prior to application to the upper body (chest, back, upper arm). In young children, the upper back is the preferred location to apply the patch, to minimize the potential of the child removing the patch.
The skin should be cleaned carefully with clean water and thoroughly dried before the transdermal patch is applied (no cleaning agents should be used!). The transdermal patch is then fixed to the skin with light pressure of the hand held flat over it (about 30 seconds). It must be ensured that the application area does not show any microlesions (e.g. due to radiation or shaving) or irritation.
As the outside of the transdermal patch is protected by a waterproof foil, the patch does not need to be removed to take a shower.
No creams, oils, lotions or powders should be used on the application area as they may impair the effective adhesion of the transdermal patch to the skin.
Duration of administration:
The transdermal patch should be changed after a period of 72 hours. If necessary in individual cases, the transdermal patch may be changed earlier but not earlier than 48 hours after application, as this could cause an increase of the mean concentration of fentanyl in serum. A new skin area must be selected for each application. However, an area of skin can be used again if a 7-day interval is observed since the removal of the last transdermal patch. The analgesic effect may persist for some time after the transdermal patch has been removed. If residue is found on the skin after the patch has been removed, it can be removed with soap and water. The cleaning should not be performed using alcohol or other solvents, as these may penetrate the skin based on the specific effects of the transdermal patch.
If progressive dose increases are made, the active skin surface area required may reach a point where no further increase is possible.
4.3 Contraindications
- hypersensitivity to the active substance, soya, peanuts or to any of the excipients listed in section 6.1.
Fenylat must not be used in the case of:
- Acute or postoperative pain, since dosage titration is not possible during short-term use and a serious or life-threatening hypoventilation may occur
- severe impairment of central nervous system
- severe respiratory depression
4.4 Special warnings and precautions for use
Patients who have experienced serious adverse events should be monitored for at least 24 hours, or longer, depending on the clinical symptoms, after removal of the patch. Serum fentanyl concentration declines gradually and is reduced by about 50% within 17 (range 13-22) hours.
Fenylat should be kept out of reach and sight of children at all times before and after use.
Do not cut Fenylat. A patch that has been divided, cut or damaged in any way should not be used.
This medicinal product should only be used under the surveillance of physicians who are experienced in pharmacokinetic of transdermal fentanyl patches and the risk of severe hypoventilation.
In case of a change of the fentanyl-containing systems, additional medical supervison and information of the patient is advised to maintain continuous reduction of pain (if necessary in the same manner as in the initial adjustment).
Respiratory depression
As with all potent opioids some patients may experience significant respiratory depression with Fenylat, and patients must be observed for these effects. Respiratory depression may persist beyond the removal of the patch. The incidence of respiratory depression increases as the fentanyl dose is higher (see section 4.9). CNS active drugs may worsen the respiratory depression (see section 4.5).
Chronic pulmonary disease
In patients with chronic obstructive or other pulmonary diseases Fenylat may have more severe adverse reactions. In such patients, opioids may decrease respiratory drive and increase airway resistance.
Drug dependence and potential for abuse
Tolerance, physical dependence and psychological dependence may develop upon repeated administration of opioids (see section 4.8). Iatrogenic addiction following opioid administration is rare. Fentanyl can be abused in a manner similar to other opioid agonists. Abuse or intentional misuse of Fenylat may result in overdose and/or death. Patients with a prior history of drug dependence/alcohol abuse are more at risk to develop dependence and abuse in opioid treatment. Patients at increased risk of opioid abuse may still be appropriately treated with modified-release opioid formulations; however, these patients will require monitoring for signs of misuse, abuse, or addiction.
Increased intracranial pressure
Fenylat should be used with caution in patients who may be particularly susceptible to the intracranial effects of CO2 retention such as those with evidence of increased intracranial pressure, impaired consciousness or coma. Fenylat should be used with caution in patients with brain tumours.
Cardiac disease
Fentanyl may produce bradycardia and Fenylat should therefore be administered with caution to patients with bradyarrhythmias.
Opioids may cause hypotension, especially in patients with acute hypovolemia. Underlying, symptomatic hypotension and/or hypovolaemia should be corrected before treatment with Fentanyl transdermal patches is initiated.
Hepatic impairment
Because Fentanyl is metabolised to inactive metabolites in the liver, hepatic impairment might delay its elimination. If patients with hepatic impairment receive Fenylat, they should be observed carefully for signs of fentanyl toxicity and the dose of Fenylat should be reduced if necessary (see section
5.2).
Renal impairment
Less than 10 % of fentanyl is excreted unchanged by the kidneys, and unlike morphine, there are no known active metabolites eliminated by the kidneys. If patients with renal impairment receive Fenylat they should be observed carefully for signs of fentanyl toxicity and the dose should be reduced if necessary.
Fever/external heat application
In a pharmacokinetic model, evidence was seen that the concentration of fentanyl in blood may possibly increase by one third, when the skin temperature rises to 40°C. For this reason, febrile patients must be closely monitored for undesirable effects and the Fenylat dose must be adapted if necessary. There is a potential for temperature-dependent increases in fentanyl released from the patch resulting in possible overdose and death. A clinical pharmacology trial conducted in healthy adult subjects has shown that the application of heat over the Fentanyl transdermal patch increased mean fentanyl AUC values by 120% and mean Cmax values by 61%. All patients should be advised to avoid exposing the Fenylat application site to direct external heat sources such as heating pads, electric blankets, heated water beds, heat or tanning lamps, intensive sun bathing, hot water bottles, saunas, prolonged hot baths or hot whirlpool spa baths while wearing the patch, since there is potential for temperature dependent increases in release of fentanyl from the patch.
Serotonin syndrome
Caution is advised when Fenylat is co-administered with drugs that effect the serotonergic neurotransmitter systems.
The development of a potentially life-threatening serotonin syndrome may occur with the concomitant use of serotonergic drugs such as Selective Serotonin Re-uptake Inhibitors (SSRIs), Serotonin Norepinephrine Re-uptake Inhibitors (SNRIs) and drugs which impair the metabolism of serotonin (including Monoamine Oxidase Inhibitors [MAOIs]). This may occur within the recommended dose.
Serotonin syndrome may include one or more of the following: mental-status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular abnormalities (e.g., hyperreflexia, incoordination, rigidity) and/or gastrointesinal symptoms (e.g., nausea, vomoting, diarrhoea).
If serotonin syndrome is suspected, rapid discontinuation of Fenylat transdermal patch should be considered.
Inadvertent exposure due to patch transfer
Inadvertent transfer of a fentanyl-containing patch to the skin of another person (especially of a child) when sharing a bed or by close physical contact with a person wearing a patch may induce opioid overdose in the person who normally does not use fentanyl patches. Patients should be advised that inadvertently transferred patches must be removed from the skin of the nonpatch wearer immediately (see section 4.9).
Breakthrough pain
Studies showed that most patients, despite a treatment with fentanyl transdermal patches, need to be treated additionally with a strong and fast releasing medicine to release from breakthrough pain.
Paediatric population
Fenylat should not be administered to opioid-naive paediatric patients (see section 4.2). The potential for serious or life-threatening hypoventilation exists regardless of the dose of Fenylat transdermal system administered.
Fenylat has not been studied in children under 2 years of age. Fenylat should be administered only to opioid-tolerant children age 2 years or older (see section 4.2). Fenylat should not be used in children under 2 years of age.
To guard against accidental ingestion by children, use caution when choosing the application site for Fenylat (see section 4.2) and monitor adhesion of the patch closely.
Elderly patients
Data from intravenous studies with fentanyl suggest that the elderly patients may be more sensitive to the drug than younger patients, with a reduced clearance and a prolonged half-life of fentanyl. If elderly patients receive Fenylat, they should be observed carefully for signs of fentanyl toxicity and the dose should be reduced if necessary (see section 5.2).
Gastrointestinal tract
Opioids increase the tone and decrease propulsive peristalsis of smooth muscles of the gastrointestinal tract. The resulting prolonged gastrointestinal passage time may be responsible for the constipating effect of fentanyl. Patients should be informed about measures to prevent constipation, and preventive use of laxatives should be considered. Special caution is required in patients with chronic constipation. In the case or suspicion of paralytic ileus the use of Fenylat must be terminated.
Patients with myasthenia gravis
Non-epileptic (myo)clonic reactions can occur. Caution should be exercised when treating patients with myasthenia gravis.
Misuse for doping purposes
The use of Fenylat may lead to a positive doping test. The use of Fenylat as a doping agent may be hazardous to the health.
4.5 Interaction with other medicinal products and other forms of interaction
Effects of sedation can be enhanced by simultaneous administration of other central depressant medicinal products, such as opioids, sedatives or hypnotics, general anaesthetics and muscle relaxants, phenothiazines, tranquilizers, sedating antihistamines or alcohol beverages. Hypoventilation, hypotension and profound sedation, coma or death may occur. Therefore, the use of any of the above mentioned concomitant drugs requires special care and observation and the dose of one or both of the medicinal products should be reduced.
CYP3A4-Inhibitors
Fentanyl, a substance with a high clearance, is rapidly and extensively metabolized mainly by Cytochrome-P450-(CYP) 3A4.
The concomitant use of transdermal fentanyl with cytochrome P450 3A4 (CYP3A4) inhibitors (e.g. ritonavir, ketoconazole, itraconazole, fluconazole, voriconazole, troleandomycin, clarithromycin, nelfinavir, nefazodone, verapamil, diltiazem, and amiodarone) may result in an increase in fentanyl plasma concentrations, which could increase or prolong both the therapeutic and adverse effects, and may cause serious respiratory depression.
In this situation, special patient care and observation are appropriate. The concomitant use of CYP3A4 inhibitors and transdermal fentanyl is not recommended, unless the patient is closely monitored.
Patients especially those receiving Fenylat and CYP3A4-inhibitors should be observed for signs of respiratory depressions. The dose should be adjusted if necessary (see section 4.4).
CYP3A4 inducers
Concomittant use with CYP3A4-inducing agents (e.g. rifampicin, carbamazepine, phenobarbital, phenytoin) may induce a decrease of fentanyl plasma concentrations and a decrease in therapeutic efficacy. This may require dose adjustment of fentanyl transdermal patch. Following withdrawal of a treatment with a CYP3A4 inducer, its effects gradually diminish. This can result in an increase of fentanyl plasma concentrations and to an increase or a prolongation of its therapeutic effects and side-effects. Severe respiratory depression may result. In this situation, specific patient supervision and dose adjustment are necessary.
Monoamine Oxidase Inhibitors (MAOI)
Fenylat is not recommended for use in patients who require the concomitant administration of an MAO inhibitor. Severe and unpredictable interactions with MAO inhibitors, involving the potentiation of opiate effects or the potentiation of serotoninergic effects, have been reported. Therefore, Fenylat should not be used within 14 days after discontinuation of treatment with MAO inhibitors.
Serotonergic drugs
The concomitant use of fentanyl with other serotonergic drugs, such as a Selective Serotonin Re-uptake Inhibitor (SSRI), or a Serotonin Norepinephrine Re-uptake Inhibitor (SNRI) or a Monoamine Oxidase Inhibitor (MAOI) may increase the risk of serotonin syndrome, a potentially life-threatening condition.
Mixed agonists/antagonists
The concomitant use of buprenorphine, nalbuphine or pentazocine is not recommended. They have high affinity to opioid receptors with relatively low intrinsic activity and therefore partially antagonise the analgesic effect of fentanyl and may induce withdrawal symptoms in opioid dependent patients.
4.6 Fertility, pregnancy and lactation
Pregnancy
There are no adequate data from the use of fentanyl in pregnant women. Studies in animals have shown some reproductive toxicity (see section 5.3). The potential risk for humans is unknown. Fentanyl crosses the placenta. Neonatal withdrawal syndrome has been reported in newborn infants with chronic maternal use of transdermal Fentanyl during pregnancy. Fenylat should not be used during pregnancy unless clearly necessary.
Use of Fenylat during childbirth (including caesarean) is not recommended because it should not be used in the management of acute or postoperative pain (see section 4.3). Moreover, because fentanyl passes through the placenta, the use of Fenylat during childbirth might result in respiratory depression in the newborn infant.
Lactation
Fentanyl is excreted into breast milk and may cause sedation and/or respiratory depression in the breast-fed infant. Breastfeeding should therefore be discontinued during treatment with Fenylat for at least 72 hours after the removal of the patch.
Fertility
In animal studies impaired fertility was observed with high doses (see section
5.3).
4.7 Effects on ability to drive and use machines
Fenylat may impair the mental and/or physical ability required to perform potentially hazardous tasks such as driving a car or operating machinery. In patients with stable
fentanyl dose adjustment (without further influence of other active ingredients), significant impairment of the ability to drive and use machines is not expected. However at the onset of treatment, upon increase of dose or upon combination with other medicinal products, reactivity may be affected in individual cases, leading to impairment of the ability to drive or use machines. Such situations should be handled with caution.
This medicine can impair cognitive function and can affect a patient’s ability to drive safely. This class of medicine is in the list of drugs included in regulations under 5a of the Road Traffic Act 1988. When prescribing this medicine, patients should be told:
• The medicine is likely to affect your ability to drive
• Do not drive until you know how the medicine affects you
• It is an offence to drive while under the influence of this medicine
• However, you would not be committing an offence (called ‘statutory defence’) if:
o The medicine has been prescribed to treat a medical or dental problem and
o You have taken it according to the instructions given by the prescriber and in the information provided with the medicine and
o It was not affecting your ability to drive safely
4.8 Undesirable effects
The safety of fentanyl patches was evaluated in 1854 adult and paediatric subjects who participated in 11 clinical trials (double-blind fentanyl transdermal patches [placebo or active control] and/or open label Fenylat [no control or active control]) conducted to improve the management of chronic malignant or non-malignant pain. All the subjects took at least 1 dose of fentanyl transdermal patches and provided safety data.
a) Summary of the safety profile
Based on pooled safety data from these clinical trials, the most commonly (>10% incidence) reported adverse drug reactions (ADRs) were (with % incidence): nausea (35.7%), vomiting (23.2%), constipation (23.1%), somnolence (15.0%), dizziness (13.1%), headache (11.8%).
The most serious undesirable effect of fentanyl is respiratory depression.
b) Tabulated summary of adverse reactions
The ADRs reported with the use of Fentanyl patches from these clinical trials, including the above-mentioned ADRs, and from post-marketing experiences are listed in the table 4.
The displayed frequency categories use the following convention:
Very common: (>1/10)
Common: (>1/100 to <1/10)
Uncommon: (>1/1000 to <1/100)
Rare: (>1/10,000 to <1/1,000)
Very rare: (<1/10,000)
Not known: (cannot be estimated from the available data)
Table 4: Undesirable side effects of fentanyl patches from clinical
studies and experiences
after bringing onto the market
|
System Organ Class |
Adverse Drug Reactions | |||||
|
Frequency Category | ||||||
|
Very common |
Common |
Uncommon |
Rare |
Very rare |
Not known | |
|
Immune System Disorders |
Hypersensitivity |
Anaphylactic shock, Anaphylactic reaction, Anaphylactoid reaction | ||||
|
Metabolism and Nutrition Disorders |
Anorexia | |||||
|
Psychiatric Disorders |
Insomnia, Depression, Anxiety, Confusional state, Hallucination |
Agitation, Disorientation, Euphoric mood | ||||
|
Nervous System Disorders |
Somnolence, Dizziness, Headache1 |
Tremor, Paraesthesia |
Hypoaesthesia, Convulsion (including clonic convulsions and grand mal convulsion), Amnesia Speech disorder | |||
|
Eye Disorders |
Conjunctivitis |
Myosis | ||||
|
Ear and Labyrinth Disorders |
Vertigo | |||||
|
Cardiac Disorders |
Palpitations, Tachycardia |
Bradycardia, Cyanosis |
Arrhythmia | |||
|
Vascular Disorders |
Hypertension |
Hypotension |
Vasodilatation | |||
|
Respiratory, Thoracic and Mediastinal Disorders |
Dyspnoea |
Respiratory depression, Respiratory distress |
Apnoea, Hypoventilation |
Bradypnoea | ||
|
Gastrointestinal Disorders |
Nausea,1 Vomiting1, Constipation1 |
Diarrhoea1, Dry mouth, Abdominal pain, Upper abdominal pain, Dyspepsia |
Ileus |
Subileus |
Painful flatulence | |
|
Skin and Subcutaneous Tissue Disorders |
Hyperhidrosis, Pruritus1, Rash, Erythema |
Eczema, Allergic dermatitis, Skin disorder, Dermatitis, Contact dermatitis | ||||
|
Musculoskeletal and Connective Tissue Disorders |
Muscle spasms |
Muscle twitching | ||||
|
Renal and Urinary |
Urinary |
Oliguria | ||||
|
Disorders |
retention |
cystalgia | ||||
|
Reproductive System and Breast Disorders |
Erectile dysfunction, Sexual dysfunction | |||||
|
General Disorders and Administration Site Conditions |
Fatigue, Peripheral, oedema Asthenia, Malaise, Feeling cold |
Application site reaction, Influenza like illness, Feeling of body temperature change, Application site hypersensitivity, Drug withdrawal syndrome2, Fever |
Application site dermatitis, Application site eczema |
1 See section 4.8 d)
2 see section 4.8 c)
In very rare cases soya oil may cause allergic reactions.
c) Description of the chosen side effects
As with other opioid analgesics, tolerance, physical dependence, and psychological dependence can develop on repeated use of Fenylat (see section
4.4).
Opioid withdrawal symptoms (such as nausea, vomiting, diarrhoea, anxiety and shivering) are possible in some patients after conversion from their previous opioid analgesic to Fenylat or if therapy is stopped suddenly (see section 4.2).
There have been reports of newborn infants experiencing neonatal withdrawal syndrome when mothers chronically used Fenylat during pregnancy (see section 4.6).
d) Paediatric subjects
The adverse event profile in children and adolescents treated with fentanyl transdermal patches was similar to that observed in adults. No risk was identified in the paediatric population beyond that expected with the use of opioids for the relief of pain associated with serious illness. There does not appear to be any paediatric-specific risk associated with Fenylat use in children as young as 2 years old when administrated as directed. Very common adverse events reported in paediatric clinical trials were fever, headache, vomiting, nausea, constipation, diarrhoea and pruritus.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card scheme at http://yellowcard.mhra.gov.uk
Overdose
4.9
Symptoms
The manifestations of fentanyl overdosage are an extension of its pharmacological actions, the most serious effect being respiratory depression.
Treatment
In case of a respiratory depression Fenylat must be removed immediately and the patient should be encouraged to breath using verbal or physical stimulation. A specific antagonist such as naloxone may then be administered, but the respiratory depression may persist longer than the effect of the antagonist. The interval between the intravenous administered antagonist doses should be carefully chosen because of the possibility of re-narcotization after removal of the patch; repeated administration or a continuous infusion of naloxone may be necessary. Sudden pain and the release of catecholamines may be the consequence of the antagonisation.
If the clinical situation warrants, a patent airway should be established and maintained, possibly with an oropharyngeal airway or endotracheal tube., Oxygen should be administered and respiration assisted or controlled, as appropriate.
Adequate body temperature and fluid intake should be maintained.
If severe or persistent hypotension occurs, hypovolemia should be considered, and the condition should be managed with appropriate parenteral fluid therapy.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: opioids; phenylpiperidine derivatives ATCCode: N02AB03
Fentanyl is an opioid analgesic which interacts in particular with the p-receptor. The most important therapeutic effects are analgesia and sedation. The serum concentrations of fentanyl leading to a minimal analgesic effect in opioid-naive patients, ranges between 0.3-1.5 ng/ml; the frequency of undesirable effects increases above serum levels of 2 ng/ml.
The concentration leading to opioid-induced undesirable effects increases with duration of exposure of the patient to fentanyl. The predisposition to a development of tolerance varies considerably from individual to individual.
5.2 Pharmacokinetic properties
Resorption
Fentanyl is continuously absorbed through the skin over a period of 72 hours following the application of the Fenylat. The rate of release is relatively constant due to the releasing polymer matrix and the diffusion of fentanyl through the layers of the skin. The concentration gradient existing between the matrix and the lower concentration in the skin drives drug release. After the initial application of Fenylat, the serum concentration of fentanyl increases gradually reaching steady state between 12 and 24 hours after application and remains relatively constant for the entire rest of a 72-hour period. The concentration in serum attained is proportional to the size of the fentanyl transdermal patch. Following repeated applications for 72 hours each, the concentration in serum reaches a steady state which is maintained at a constant level for subsequent applications of patches of the same size.
A pharmacokinetic model has suggested that serum fentanyl concentrations may increase by 14% (range 0- 26%) if a new patch is applied after 24 hours rather than the recommended 72-hour application.
Distribution
The plasma protein binding for fentanyl is about 84 %.
Biotransformation
Fentanyl is a high clearance drug and is rapidly and extensively metabolised primarily by CYP3A4 in the liver. The major metabolite, norfentanyl, is inactive. Skin does not appear to metabolise fentanyl delivered transdermally. This was determined in a human keratinocyte cell assay and in clinical studies in which 92% of the dose delivered from the system was accounted for as unchanged fentanyl that appeared in the systemic circulation.
Elimination
After the removal of the transdermal patch containing fentanyl, serum fentanyl concentrations decline gradually, falling with a half-life time of about 17 (range 13-22) hours for adults and 22 - 25 hours in children following a 24-hour application. In another study the mean half-life ranges from 20-27 hours following a 72-hour application.Continued absorption of fentanyl from the skin accounts for a slower disappearance of the drug from the serum than is seen after an IV infusion, where the apparent half-life is approximately 7 (range 3-12) hours. Within 72 hours of IV fentanyl administration, approximately 75% of the fentanyl dose is excreted into the urine, mostly as metabolites, with less than 10% as unchanged drug. About 9% of the dose is recovered in the faeces, primarily as metabolites.
Special populations :
Elderly
Data from intravenous studies with fentanyl suggest that elderly patients may have reduced clearance, a prolonged half-life, and they may be more sensitive to the drug than younger patients. In a study conducted with fentanyl transdermal patches, healthy elderly subjects had fentanyl pharmacokinetics which did not differ significantly from healthy young subjects, although peak serum concentrations tended to be lower and mean half-life values were prolonged to approximately 34 hours. Elderly patients should be observed carefully for signs of fentanyl toxicity and the dose reduced if necessary (see section 4.4).
Paediatric Patients
The safety of Fentanyl transdermal patches was evaluated in 289 paediatric subjects (<18 years) who participated in 3 clinical trials for the management of chronic or continuous pain of malignant or non-malignant origin. These subjects took at least one dose of Fentanyl and provided safety data. Although the enrolment criteria for the paediatric studies restricted enrolment to subjects who were a minimum of 2 years of age, 2 subjects in these studies received their first dose of Fentanyl at an age of 23 months.
Fenylat has not been studied in children under 2 years. Investigations in older children with dose adaptation according to body weight demonstrated that clearance in paediatric patients is approximately 20% higher compared to adults. These findings have been taken into consideration in determining the dosing recommendations for paediatric patients. Fenylat should be administered only to opioid-tolerant paediatric patients (ages 2 to 16 years) (see sections 4.2 and 4.4).
Hepatic impairment
In a study conducted in patients with hepatic cirrhosis, the pharmacokinetics of a single 50 micrograms/h application was assessed. Although tmax and tJ/2 were not altered, the mean plasma Cmax and AUC values increased by approximately 35% and 73%, respectively, in these patients. Patients with hepatic impairment should be observed carefully for signs of fentanyl toxicity and the dose reduced if necessary (see section 4.4).
Renal impairment
Data obtained from a study administering intravenous fentanyl in patients undergoing renal transplantation suggest that the clearance of fentanyl may be reduced in this patient population. If patients with renal impairment receive fentanyl transdermal patches, they should be observed carefully for signs of fentanyl toxicity and the dose reduced if necessary (see section 4.4).
The transdermal patch is an application form for the systemic administration of fentanyl, which ensures that an adequate serum level of fentanyl will be maintained over a period of 72 hours with a constant rate of release.
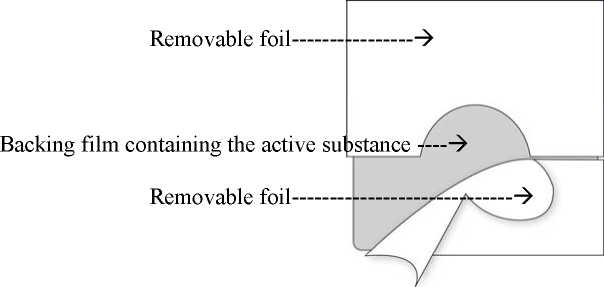
The transdermal patch consists of two functional layers:
The topside consists of a waterproof backing film with a selfadhesive fentanyl-containing matrix layer on it. This matrix layer is covered by a removable foil which - due to slits - can be easily removed prior to use.
Corresponding to the different sizes of absorption surfaces of the five systems of 4.25, 8.5, 17, 25.5 and 34 cm2 approximately 12, 25, 50, 75 or 100 micrograms/hour fentanyl are released to the skin. This is achieved by means of the polymer matrix: A concentration gradient is created between the polymer matrix with high concentrations of the active substance fentanyl and the skin with low concentration of fentanyl. Over a period of 72 hours fentanyl diffuses towards the lower concentration, i.e. towards the skin.
The relative bioavailability of fentanyl from the patch is 92%. The serum fentanyl concentrations attained are proportional to the patch size.
Preclinical safety data
5.3
Based on conventional studies on safety pharmacology, toxicity, repeated dose, genotoxicity and carcinogenic potential, preclinical data do not indicate specials risk for humans.
In a study in rats no influence on male fertility was seen. In another study on fertility and early embryonic development in rats after high doses (300 micrograms/kg/day s.c.), an effect mediated by male animals was observed which was presumably related to the sedative effects of fentanyl in animal experiments. Investigations in female rats showed reduced fertility and embryonic mortality. Newer studies demonstrated that the embryotoxic effects are indirectly induced by maternal toxicity and are not based on a direct effect of the active substance on the developing embryo. Investigations in two species did not indicate teratogenic effects. In a pre- and postnatal study, survival rate of the offsprings was significantly lowered on day 4 of the lactation period when doses were administered that induced a mild reduction in maternal body weight. In a study investigating developmental and reproduction toxicity in rats receiving maternally toxic doses of fentanyl, a delay in physical development, sensory functions, reflexes and behavior of offsprings was observed. These effects may be due to changes in brood caring by the dam or to an indirect effect of fentanyl on the offsprings.
In a two-year carcinogenicity study conducted in rats, fentanyl was not associated with an increased incidence of tumours at subcutaneous doses up to 33 micrograms/kg/day in males or 100 micrograms/kg/day in females (corresponding to the 0.16- and 0.39-fold of the daily human dose achieved by the 100 micrograms/h patch, based on a comparison of AUC0_24h).
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Matrix Components:
Aloe vera leaf extract oil (on the basis of soya oil tocopherol acetate) Colophonium resin Poly (2-ethylhexylacrylate,vinylacetate) (50:50)
Release liner:
Polyethylene terephtalat, polyester, siliconized
Backing foil with imprint:
Polyethylene terephthalat foil,
Printing ink
6.2
Incompatibilities
To prevent interference with the adhesive properties of the patch, no creams, oils, lotions or powder should be applied to the skin area when the patch is applied.
6.3 Shelf life
2 years.
6.4 Special precautions for storage
This medicinal product does not require any special storage conditions.
6.5 Nature and contents of container
Each transdermal patch is packed individually into a sealed child resistant sachet. The sachet is composed of different layers, polyester, aluminium foil and surlyn and is tightly sealed.
5, 10, 20 transdermal patches Not all pack sizes may be marketed.
6.6 Special precautions for disposal
Note for handling:
See section 4.2
Note for disposal:
Used transdermal patches should be folded with the adhesive surfaces inwards and discarded safely or whenever possible returned to the pharmacy. Patches which have not been used should be disposed of after consultation with the pharmacist.
Other notes:
Fenylat is exclusively for use on the skin of persons to whom it was prescribed. In rare cases, the patch could be attached to another person’s skin following close physical contact. In such a case the patch has to be removed immediately.
Please wash hands after application or removal of a patch (do not use cleansing or soap-like products).
7 MARKETING AUTHORISATION HOLDER
Activase Pharmaceuticals Limited,
11 Boumpoulinas, P.C.1060 Nicosia.
Cyprus
8 MARKETING AUTHORISATION NUMBER(S)
PL 28444/0128
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
18/02/2014
10 DATE OF REVISION OF THE TEXT
18/02/2014