Fliveo Inhaler 250 Micrograms Per Actuation Pressurised Inhalation Suspension
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Fliveo Inhaler 250 micrograms per actuation pressurised inhalation, suspension
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Each metered dose (ex-valve) contains 250 micrograms fluticasone propionate. This is equivalent to a delivered dose (ex-actuator) of 220 micrograms fluticasone propionate.
For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Pressurised inhalation, suspension.
Fliveo Inhaler is supplied in a pressurised aluminium multidose canister, fitted with a metering valve.
The canister contains a white to off-white suspension.
The canisters are fitted into dark brown plastic actuators fitted with white dust caps.
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Fluticasone propionate given by inhalation offers prophylactic treatment for mild, moderate and severe asthma.
Adults and adolescents over 16years:
Fliveo Inhaler 250 micrograms is indicated in adults and adolescents over 16 years of age for the prophylactic treatment of asthma.
Children aged over 4 years up to 12 years and adolescents aged 13 to 16 years:
Only the lowest strength of this inhaled corticosteroid, Fliveo Inhaler 50 micrograms, is authorised for use in these young age groups.
Fliveo Inhaler 50 micrograms is indicated in any child (aged over 4 years up to 12 years) and adolescents (aged 13 to 16 years) with asthma and requiring prophylactic treatment, including those not controlled currently on other available prophylactic treatments.
Fliveo Inhaler 125 micrograms and Fliveo Inhaler 250 micrograms are NOT authorised for use in these young age groups.
Asthma severity
Mild asthma: Patients requiring intermittent symptomatic rapid-acting bronchodilators (p2 agonists) on a regular daily basis.
Moderate persistent asthma: Patients with daily symptoms, daily use of ‘rescue’ rapid-acting bronchodilators (p2 agonists) and moderate to severe airflow limitation and for whom rapid control of asthma is essential.
Severe asthma: Patients with severe chronic asthma and those who are dependent on systemic corticosteroids for adequate control of symptoms. Introduction of inhaled fluticasone propionate may allow for significant reduction, or elimination, of oral corticosteroid use (see section 4.4).
4.2 Posology and method of administration
Route of administration: Inhalation use.
Patients should be made aware of the prophylactic nature of therapy with inhaled fluticasone propionate and that it should be used regularly, even when asymptomatic. The onset of therapeutic effect is within 4 to 7 days.
If patients find that relief with rapid-acting bronchodilator treatment becomes less effective, or they need more inhalations than usual, medical attention must be sought (see section 4.4).
Patients should be given a starting dose of inhaled fluticasone propionate which is appropriate to the severity of their disease.
Dose titration
The dose may be increased until control is achieved. The dose should then be titrated down to the lowest dose at which effective control of asthma is maintained, according to the individual response.
Posology
Adults and adolescents over 16years:
Patients should be given a starting dose of inhaled fluticasone propionate which is appropriate for the severity of their disease. The dose should then be individually adjusted until control is achieved; the dose may be increased to achieve control of asthma but should then be titrated downwards to the lowest dose at which effective control of asthma is maintained.
Usual doses are between 100 micrograms to a maximum of 1,000 micrograms, twice daily.
Typical starting doses for adults and adolescents over 16 years: Mild asthma: Typical starting dose is 100 micrograms twice daily.
Moderate and more severe asthma: Starting doses may need to be 250 to 500 micrograms twice daily.
However due to the risk of systemic effects, doses above 500 micrograms twice daily should be prescribed only for adult patients with severe asthma where additional clinical benefit is expected, demonstrated by either an improvement in pulmonary function and/or symptom control, or by a reduction in oral corticosteroid therapy (see sections 4.4 and 4.8).
Therefore in severe asthma where additional clinical benefit is expected: Doses of up to 1,000 micrograms twice daily may be used. Initiation of such doses should be prescribed only by a specialist in the management of asthma (such as a consultant physician or general practitioner with appropriate experience).
Paediatric population - children aged over 4 years up to 12 years and adolescents aged 13 to 16 years:
Only the lowest strength of this inhaled corticosteroid, Fliveo Inhaler 50 micrograms, is authorised for use in these young age groups.
Fliveo Inhaler 125 micrograms and Fliveo Inhaler 250 micrograms are NOT authorised for use in these age groups; the maximum dose authorised for use in children aged over 4 years up to 12 years and adolescents aged 13 to 16 years is 200 micrograms twice daily (4 x 50 micrograms twice daily, a dose which CANNOT be prescribed using the two higher strengths of this inhaled corticosteroid).
Special patient groups
There is no need to adjust the dose in elderly patients or in those with hepatic or renal impairment.
Use of spacer device
A Volumatic® spacer device should always be available for use with a pressurised metered dose inhaler and should always be considered for use when a pressurised metered dose inhaler is prescribed for use by a child, and may need to be used with a face mask.
Fliveo Inhaler may also be used with a Volumatic® spacer device by patients who find it difficult to synchronise aerosol actuation with inspiration of breath, or those administering doses above 1,000 micrograms (500 micrograms twice daily) to help reduce side effects in the mouth and throat (see section 4.4).
The spacer device must be appropriate for the age groups of intended use.
Only the Volumatic® spacer device should be used with Fliveo Inhaler. Other spacing devices should not be used with Fliveo Inhaler and patients should not switch from one spacer device to another.
Patients should be instructed in the proper use and care of their inhaler and spacer device. Technique when using the inhaler both without and with the spacer device should be checked regularly to ensure that actuation of the inhaler and inspiration of breath are synchronised and to ensure optimum delivery of the inhaled drug to the lungs. Patients should always use the recommended Volumatic® spacer device as switching to an alternative spacer device can result in changes in the dose of fluticasone propionate delivered to the lungs. Patients should also be told not to discontinue their use of a spacer device without consulting their doctor or asthma nurse, as a reduction in the dose delivered to the lungs may be expected (see section 4.4).
Re-titration to the lowest dose at which effective control of asthma is maintained should always be carried out when patients who have not used a spacer device previously are prescribed the Volumatic® spacer device and when patients who have been using an alternative product without or with a spacer device are transferred to Fliveo Inhaler, used either without or with the Volumatic® spacer device.
Method of administration
Instructions for use
Patients should be instructed in the proper use of their inhaler (see package leaflet). During inhalation, the patient should preferably sit or stand.
The inhaler has been designed for use in a vertical position.
Testing the inhaler
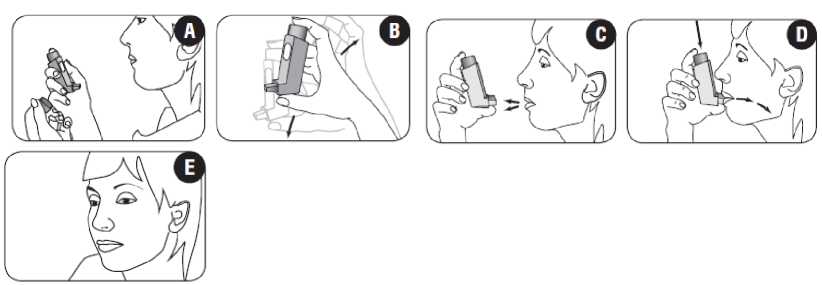
Before using the inhaler for the first time patients should test that it is working. Patients should remove the mouthpiece cover by gently squeezing the sides of the cover (figure A) and hold the inhaler between the fingers and thumb with their thumb on the base, below the mouthpiece (figure B). To make sure that the inhaler works, the patient should shake it well, point the mouthpiece away from them and press the canister firmly to release an actuation (puff) into the air. These steps should be repeated at least two times, shaking the inhaler before releasing each puff.
If the inhaler has not been used for one day (24 hours) or more, the mouthpiece cover should be removed, the patient should shake the inhaler well and two puffs should be released into the air to prime the inhaler.
If the inhaler is cold, the canister should be removed from the actuator and warmed in the hands. No other form of external heat should be applied.
Use of the inhaler
1. Patients should remove the mouthpiece cover by gently squeezing the sides of the cover (figure A).
2. Patients should check inside and outside of the inhaler including the mouthpiece for the presence of loose objects (figure A).
3. Patients should shake the inhaler well prior to use to ensure that any loose objects are removed and that the contents of the inhaler are evenly mixed (figure B).
4. Patients should hold the inhaler upright between the fingers and thumb with their thumb on the base, below the mouthpiece (figure C).
5. Patients should breathe out as far as is comfortable and then place the mouthpiece in their mouth between their teeth and close their lips around it. Patients should be instructed not to bite the mouthpiece (figure D).
6. Just after starting to breathe in through their mouth, patients should press firmly down on the top of the inhaler to release the medicine whilst still breathing in steadily and deeply (figure D).
While holding their breath, patients should take the inhaler from their mouth and take their finger from the top of the inhaler. Patients should continue holding their breath for as long as is comfortable (figure E).
7.
8. 9.
To take a second inhalation, patients should keep the inhaler upright and wait about half a minute before repeating steps 3 to 7.
Patients should immediately replace the mouthpiece cover by firmly pushing and snapping the cap into position. This does not require excessive force, the cover should click into position.
IMPORTANT
Patients should not rush stages 5, 6 and 7. It is important that patients start to breathe in as slowly as possible just before operating their inhaler.
Patients should practise in front of a mirror for the first few times. If they see "mist" coming from the top of their inhaler or the sides of their mouth they should start again from stage 3.
Patients should rinse their mouth out with water and spit out, and/or brush their teeth after each dose of medicine, in order to minimise the risk of oropharyngeal candidiasis and hoarseness.
Patients with weak hands, or children, may find it easier to operate the inhaler with both hands, by putting both forefingers on the top of the inhaler, and both thumbs on the bottom below the mouthpiece. Younger children may need help from a parent or carer when using the inhaler.
Cleaning the inhaler (also detailed in package leaflet)
The inhaler should be cleaned at least once a week. The mouthpiece cover should be removed and then replaced when cleaning is complete. The inside and outside of the mouthpiece and plastic casing should be wiped with a dry cloth. The canister should not be removed, nor placed in water. Before use, one puff should be released into the air (see package leaflet).
THE METAL CANISTER SHOULD NOT BE PUT IN WATER.
4.3
Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1.
4.4 Special warnings and precautions for use
Management of asthma
The management of asthma should follow a stepwise programme, and patient response should be monitored clinically and by lung function tests.
Once asthma symptoms are controlled, consideration may be given to gradually reducing the dose of fluticasone propionate. Regular review of patients as treatment is stepped down is important. The lowest dose at which effective control of asthma is maintained should be used (see section 4.2).
Fluticasone propionate should not be used to treat acute asthma symptoms for which an inhaled rapid-acting bronchodilator (e.g. p2 agonist) is required. Patients should be advised to have their inhaler to be used for relief in an acute asthma attack available at all times.
Severe asthma requires regular medical assessment, including lung function testing, as patients are at risk of severe attacks and even death. Increasing symptoms may indicate deterioration of asthma control and medical assessment may be required.
Treatment with fluticasone propionate should not be stopped abruptly due to the risk of exacerbation. Therapy should be down-titrated under medical supervision.
Paradoxical bronchospasm
As with other inhalation therapy, paradoxical bronchospasm may occur with an immediate increase in wheezing and shortness of breath after dosing. Paradoxical bronchospasm responds to a rapid-acting bronchodilator and should be treated straightaway. Fluticasone propionate should be discontinued immediately, the patient assessed and alternative therapy instituted if necessary.
Deterioration of asthma control
Serious asthma-related adverse events and exacerbations may occur during treatment with fluticasone propionate. Patients should be asked to continue treatment but to seek medical advice if symptoms remain uncontrolled or worsen on initiation on Fliveo Inhaler.
Increased requirements for use of reliever medication (rapid-acting bronchodilators), or decreased response to reliever medication, indicate deterioration of asthma control and patients should be reviewed by a physician.
Sudden and progressive deterioration, or lack of response, in asthma control is potentially life-threatening and the patient should undergo urgent medical assessment. Consideration should be given to increasing corticosteroid therapy (e.g. higher doses of inhaled corticosteroids or a course of oral corticosteroids). In patients considered at risk, daily peak flow monitoring may be instituted.
Severe exacerbations of asthma must be treated in the normal way.
If necessary, an antibiotic should be considered if there is a concomitant bacterial infection.
Inhaler technique
Patients’ inhaler technique should be checked regularly to make sure that inhaler actuation is synchronised with inspiration to ensure optimum delivery to the lungs (see section 4.2 ‘Use of the inhaler’).
Blood glucose/patients with diabetes mellitus
There have been very rare reports of increases in blood glucose levels, in patients with or without a history of diabetes mellitus (see section 4.8). This should be considered in particular when prescribing to patients with a history of diabetes mellitus.
Hypothalamic-pituitary-adrenal (HPA) axis suppression
Systemic effects may occur with any inhaled corticosteroid, particularly at high doses prescribed for long periods. These effects are much less likely to occur than with oral corticosteroids. Certain individuals, however, can show greater susceptibility to the effects of inhaled corticosteroids.
Possible systemic effects include Cushing's syndrome, Cushingoid features, adrenal suppression, acute adrenal crisis, decrease in bone mineral density, cataract and glaucoma and more rarely, a range of psychological or behavioural effects including psychomotor hyperactivity, sleep disorders, anxiety, depression or aggression (particularly in children) (see Paediatric population sub-heading below for information on the systemic effects of inhaled corticosteroids in children and adolescents).
It is important therefore, that the patient is reviewed regularly and the dose of inhaled corticosteroid is reduced to the lowest dose at which effective control of asthma is maintained.
Prolonged treatment of patients with high doses of inhaled corticosteroids may result in adrenal suppression and acute adrenal crisis. Very rare cases of adrenal suppression and acute adrenal crisis have also been described with doses of fluticasone propionate between 500 and less than 1,000 micrograms. Situations, which could potentially trigger acute adrenal crisis, include trauma, surgery, infection or any rapid reduction in dosage.
Presenting symptoms are typically vague and may include anorexia, abdominal pain, weight loss, tiredness, headache, nausea, vomiting, hypotension, decreased level of consciousness, hypoglycaemia, and seizures.
Additional systemic corticosteroid cover should be considered during periods of stress or elective surgery.
Oral corticosteroid therapy
The benefits of inhaled fluticasone propionate therapy should minimise the need for oral corticosteroids, but patients transferring from oral corticosteroids, particularly after prolonged or high dose treatment, may remain at risk of impaired adrenal reserve and associated adverse effects for a considerable time. Therefore these patients should be treated with special care and adrenocortical function regularly monitored. Patients who have required high dose emergency corticosteroid therapy in the past may also be at risk.
Where impaired adrenal reserve is identified, patients should carry a steroid warning card or notify the Doctor that they are taking fluticasone propionate as they may require appropriate supplementary systemic corticosteroid treatment during periods of stress e.g. worsening asthma attacks, chest infections, major concurrent illness, surgery and trauma.
The possibility of residual impairment should always be borne in mind in emergency and elective situations also likely to produce stress for all patients, and appropriate corticosteroid treatment must be considered. The extent of the adrenal impairment may require specialist advice before elective procedures.
Gradual and cautious withdrawal of the oral corticosteroid should commence with decreases in dose appropriate to the level of maintenance oral corticosteroid (for more information, refer to the SmPC of the oral corticosteroid).
Some patients feel unwell in a non-specific way during the withdrawal phase despite maintenance or even improvement of respiratory function. They should be encouraged to persevere with inhaled fluticasone propionate and to continue withdrawal of the oral corticosteroid, unless there are objective signs of adrenal insufficiency.
Use of a spacer device
Administration of high doses, above 1,000 micrograms daily is recommended through a Volumatic® spacer device to reduce side effects in the mouth and throat.
However, as systemic absorption of fluticasone propionate is largely through the lungs, the use of a spacer device with a metered dose inhaler may increase drug delivery to the lungs. It should be noted that this could potentially lead to an increase in the risk of systemic adverse effects. A lower dose may be required (see section 4.2).
Exacerbation of underlying conditions
Replacement of systemic corticosteroid treatment with inhaled therapy sometimes unmasks allergies such as allergic rhinitis or eczema previously controlled by the systemic drug. These allergies should be symptomatically treated with antihistamine and/or topical preparations, including topical corticosteroids.
Concomitant infections
As with all inhaled corticosteroids, fluticasone propionate should be administered with caution in patients with active or quiescent pulmonary tuberculosis and fungal, viral or other infections of the airway. Appropriate treatment should be instituted promptly, if indicated and patients observed closely.
Ritonavir
Ritonavir can greatly increase the concentration of fluticasone propionate in plasma. Therefore, concomitant use should be avoided, unless the potential benefit to the patient outweighs the risk of systemic corticosteroid side effects. There is also an increased risk of systemic side effects when combining fluticasone propionate with other potent CYP3A inhibitors (see section 4.5).
Paediatric population
Children and adolescents 16 years of age and younger inhaling high doses of fluticasone propionate (typically > 1,000 micrograms/day) may be at particular risk of systemic effects. Systemic effects may occur, particularly at high doses prescribed for long periods. Possible systemic effects include Cushing's syndrome, Cushingoid features, adrenal suppression, acute adrenal crisis and growth retardation in children and adolescents and more rarely, a range of psychological or behavioural effects including psychomotor hyperactivity, sleep disorders, anxiety, depression or aggression. Consideration should be given to referring the child or adolescent to a paediatric respiratory specialist.
It is recommended that the height of children receiving prolonged treatment with an inhaled corticosteroid is regularly monitored. If growth is slowed, therapy should be reviewed and the dose of inhaled corticosteroid should be reduced, if possible, to the lowest dose at which effective control of asthma is maintained.
A Volumatic® spacer device should always be considered for use when a pressurised metered dose inhaler is prescribed for use in this population, and may need to be used with a face mask for younger children (see section 4.2).
4.5 Interaction with other medicinal products and other forms of interaction
Under normal circumstances, low plasma concentrations of fluticasone propionate are achieved after inhaled dosing, due to extensive first pass metabolism and high systemic clearance mediated by cytochrome P450 3A4 in the gut and liver. Hence, clinically significant drug interactions mediated by fluticasone propionate are unlikely.
In an interaction study in healthy subjects with intranasal fluticasone propionate, ritonavir (a highly potent cytochrome P450 3A4 inhibitor) 100 mg b.i.d. increased the fluticasone propionate plasma concentrations several hundred fold, resulting in markedly reduced serum cortisol concentrations. Information about this interaction is lacking for inhaled fluticasone propionate, but a marked increase in fluticasone propionate plasma levels is expected. Cases of Cushing's syndrome and adrenal suppression have been reported. The combination should be avoided unless the benefit outweighs the increased risk of systemic glucocorticoid side effects.
In a small study in healthy volunteers, the slightly less potent CYP3A inhibitor ketoconazole increased the exposure of fluticasone propionate after a single inhalation by 150%. This resulted in a greater reduction of plasma cortisol as compared with fluticasone propionate alone. Co-treatment with other potent CYP3A inhibitors, such as itraconazole, and moderate CYP3A inhibitors, such as erythromycin, is also expected to increase the systemic fluticasone propionate exposure and the risk of systemic side effects. Caution is recommended and longterm treatment with such drugs should, if possible, be avoided.
4.6 Fertility, pregnancy and lactation
Fertility
There are no data in humans. No evidence of impairment of fertility was observed in reproductive studies conducted in male and female rats (see section 5.3).
Pregnancy
There is inadequate evidence of safety of fluticasone propionate in human pregnancy.
Data on a limited number (between 300 and 1000 pregnancy outcomes) of exposed pregnancies indicate no adverse effects of fluticasone propionate on pregnancy or the health of the fetus/new born child. To date no other relevant epidemiological data are available.
Administration of corticosteroids to pregnant animals have shown reproductive toxicity (see section 5.3). There may therefore be a very small risk of such effects in the human fetus. It should be noted, however, that the fetal changes in animals occur after relatively high systemic exposure. Fliveo Inhaler delivers fluticasone propionate directly to the lungs by the inhaled route and therefore, the high level of exposure that occurs when corticosteroids are given by systemic routes is avoided.
Administration of fluticasone propionate during pregnancy should only be considered if the expected benefit to the mother is greater than any possible risk to the fetus.
The lowest effective dose of fluticasone propionate needed to maintain adequate asthma control should be used in the treatment of pregnant women.
Breastfeeding
The secretion of fluticasone propionate in human breast milk has not been investigated.
Subcutaneous administration of fluticasone propionate to lactating laboratory rats produced measurable plasma levels and evidence of fluticasone propionate in the milk. However, plasma levels in humans after inhalation at recommended doses are likely to be low.
A risk to breastfed newborns/infants cannot be excluded. A decision must be made whether to discontinue breastfeeding or to discontinue treatment with fluticasone propionate taking into account the benefit of breastfeeding for the child and the benefit of fluticasone propionate for the woman.
4.7 Effects on ability to drive and use machines
Fluticasone propionate has no or negligible influence on the ability to drive and use machines.
4.8 Undesirable effects
Adverse events are listed below by system organ class and frequency. Frequencies are defined as: very common (>1/10), common (>1/100 and <1/10), uncommon (>1/1,000 and <1/100), rare (>1/10,000 and <1/1,000), very rare (<1/10,000) and not known (cannot be estimated from the available data) including isolated reports.
Very common, common and uncommon events were generally determined from clinical trial data. Rare and very rare events were generally determined from spontaneous data._
|
System Organ Class |
Adverse Event |
Frequency |
|
Infections and infestations |
Candidiasis of the mouth and throat* |
Very common |
|
Pneumonia (in COPD patients)**** |
Common | |
|
Oesophageal candidiasis* |
Rare | |
|
Immune system disorders |
Hypersensitivity reactions with the following manifestations: | |
|
Cutaneous hypersensitivity reactions |
Uncommon | |
|
Angioedema (mainly facial and oropharyngeal oedema) |
Very rare | |
|
Respiratory symptoms (dyspnoea and/or bronchospasm) |
Very rare | |
|
Anaphylactic reactions |
Very rare |
|
Endocrine disorders |
Cushing's syndrome, Cushingoid features, adrenal suppression, growth retardation in children and adolescents, decreased bone mineral density, cataract, glaucoma** |
Very rare |
|
Metabolism and nutrition disorders |
Hyperglycaemia (see section 4.4) |
Very rare |
|
Psychiatric disorders |
Anxiety, sleep disorders, behavioural changes, including hyperactivity and irritability (predominantly in children)** |
Very rare |
|
Depression, aggression (predominantly in children)** |
Not known | |
|
Respiratory, thoracic and mediastinal disorders |
Hoarseness/dysphonia* |
Common |
|
Paradoxical bronchospasm*** |
Very rare | |
|
Epistaxis |
Not known | |
|
Gastrointestinal disorders |
Dyspepsia |
Very rare |
|
Skin and subcutaneous tissue disorders |
Contusions |
Common |
|
Musculoskeletal and connective tissue disorders |
Arthralgia |
Very rare |
*Hoarseness and candidiasis (thrush) of the mouth and throat and, rarely, of the oesophagus can occur in some patients. Both hoarseness and candidiasis of the mouth and throat may be relieved, or their incidence may be reduced, by rinsing the mouth with water and/or brushing the teeth after using Fliveo Inhaler. Symptomatic mouth and throat candidiasis can be treated with topical anti-fungal therapy whilst still continuing with Fliveo Inhaler.
**Possible systemic effects include Cushing's syndrome, Cushingoid features, adrenal suppression, growth retardation, decreased bone mineral density, cataract, glaucoma, anxiety, sleep disorders, behavioural changes, including hyperactivity, irritability and depression and aggression (predominantly in children) (see section 4.4).
***As with other inhalation therapy, paradoxical bronchospasm may occur (see section 4.4) with an immediate increase in wheezing and shortness of breath after dosing. Paradoxical bronchospasm responds to a rapid-acting bronchodilator and should be treated straightaway. Fluticasone propionate should be discontinued immediately, the patient assessed, and if necessary alternative therapy instituted.
****There was an increased reporting of pneumonia in studies of patients with Chronic Obstructive Pulmonary Disease (COPD) receiving 500 micrograms fluticasone propionate. Physicians should remain vigilant for the possible development of pneumonia in patients being treated with fluticasone propionate as the clinical features of pneumonia and exacerbation frequently overlap.
Paediatric population
Possible systemic effects include Cushing's syndrome, Cushingoid features, adrenal suppression and growth retardation in children and adolescents (see section 4.4).
Children may also experience anxiety, sleep disorders and behavioural changes, including hyperactivity, irritability, depression and aggression.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the MHRA’s Yellow Card Scheme (https://yellowcard.mhra.gov.uk).
4.9 Overdose
Acute: Inhalation of fluticasone propionate in doses in excess of those recommended may lead to temporary suppression of adrenal function. This does not necessitate emergency action being taken. Adrenal function recovers in a few days and can be verified by measuring plasma cortisol.
Chronic overdose of inhaled fluticasone propionate: If higher than approved doses are continued over prolonged periods, significant adrenocortical suppression is possible.
There have been very rare reports of acute adrenal crisis occurring in adults and in children. Observed features include hypoglycaemia, decreased consciousness and/or convulsions. Situations which could potentially trigger acute adrenal crisis include exposure to trauma, surgery, infection or any rapid reduction in dose of the inhaled corticosteroid.
Adrenal reserve should be monitored and treatment with a systemic corticosteroid may be necessary. When stabilised, treatment should be continued with an inhaled corticosteroid at the recommended dose (see section 4.4 'Hypothalamic-pituitary-adrenal (HPA) axis suppression" and 'Paediatric population’).
Treatment
Patients receiving higher than approved doses should be managed closely and the dose reduced gradually.
In cases of both acute and chronic fluticasone propionate overdose, fluticasone propionate therapy should be continued at a suitable dosage for symptom control.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic group: Other drugs for obstructive airway diseases, inhalants, glucocorticoids, ATC code: R03BA05
Fluticasone propionate given by inhalation at recommended doses has a potent glucocorticoid anti-inflammatory action within the lungs, resulting in a reduction of both symptoms and exacerbations of asthma, with a lower incidence and severity of adverse effects than those observed when corticosteroids are administered systemically.
5.2 Pharmacokinetic properties
Absorption
The absolute bioavailability of a single dose of inhaled fluticasone propionate in healthy subjects varies between approximately 5 to 11% of the nominal dose depending on the inhalation device used. In patients with asthma a lesser degree of systemic exposure to inhaled fluticasone propionate has been observed.
Systemic absorption occurs mainly through the lungs and is initially rapid then prolonged. The remainder of the inhaled dose may be swallowed but contributes minimally to systemic exposure due to the low aqueous solubility and pre-systemic metabolism, resulting in oral availability of less than 1%. There is a linear increase in systemic exposure with increasing inhaled dose.
Distribution
The disposition of fluticasone propionate is characterised by high plasma clearance (1150 mL/min), a large volume of distribution at steady-state (approximately 300 L) and a terminal half-life of approximately 8 hours.
Plasma protein binding is 91%.
Biotransformation
Fluticasone propionate is cleared very rapidly from the systemic circulation. The main pathway is metabolism to an inactive carboxylic acid metabolite, by the cytochrome P450 enzyme CYP3A4. Other unidentified metabolites are also found in the faeces.
Elimination
The renal clearance of fluticasone propionate is negligible. Less than 5% of the dose is excreted in urine, mainly as metabolites. The main part of the dose is excreted in faeces as metabolites and unchanged drug.
5.3 Preclinical safety data
The only safety concerns for human use derived from animal studies of fluticasone propionate were effects associated with exaggerated pharmacological actions, and these only occurred at doses greatly in excess of that proposed for therapeutic use.
In animal reproduction studies, glucocorticosteroids have been shown to induce malformations (including growth retardation, cleft palate and skeletal malformations). These animal experimental results do not seem to be relevant for (adult) man given recommended doses; however very rarely, growth retardation may occur in children inhaling fluticasone propionate (see section 4.8). No novel effects were identified in repeat dose toxicity tests, reproductive studies or teratology studies. Fluticasone propionate is devoid of mutagenic activity in vitro and in vivo and showed no tumorigenic potential in rodents. It is both non-irritant and non-sensitising in animal models.
The non-CFC propellant, norflurane (HFA 134a), has been shown to have no toxic effect at very high vapour concentrations, far in excess of those likely to be experienced by patients, in a wide range of animal species exposed daily for periods of two years.
The use of norflurane (HFA 134a) as a propellant has not altered the toxicity profile of fluticasone propionate compared to that using the conventional CFC propellant.
6 PHARMACEUTICAL PARTICULARS
6.1 List of excipients
Propellant: norflurane (HFA 134a).
6.2 Incompatibilities
Not applicable.
6.3 Shelf life
24 months.
6.4 Special precautions for storage
Store below 25°C. Do not refrigerate or freeze. Protect from frost and direct sunlight.
As with most inhaled medicinal products in pressurised canisters, the therapeutic effect of this medicinal product may decrease when the canister is cold.
The multidose canister contains a pressurised liquid. Do not expose to temperatures higher than 50°C. Do not pierce, break or burn the canister, even if apparently empty.
Replace the mouthpiece cover firmly and snap into position after use.
6.5 Nature and contents of container
Pressurised aluminium multidose canister sealed with a metering valve, with a polypropylene actuator and a polypropylene dust cap.
Fliveo Inhaler 250 micrograms has a dark brown actuator and a white dust cap.
Each canister contains 120 metered actuations of 250 micrograms of fluticasone propionate.
6.6 Special precautions for disposal
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
7 MARKETING AUTHORISATION HOLDER
Prosonix Limited
The Magdalen Centre
The Oxford Science Park
Oxford
OX4 4GA
UK
8 MARKETING AUTHORISATION NUMBER(S)
PL 41731/0003
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
16/12/2015
10 DATE OF REVISION OF THE TEXT
16/12/2015