Fliveo Inhaler 50 Micrograms Per Actuation Pressurised Inhalation Suspension
PACKAGE LEAFLET: INFORMATION FOR THE PATIENT
Fliveo® Inhaler 50 micrograms per actuation pressurised inhalation, suspension Fliveo® Inhaler 125 micrograms per actuation pressurised inhalation, suspension Fliveo® Inhaler 250 micrograms per actuation pressurised inhalation, suspension
fluticasone propionate
Read all of this leaflet carefully before you start using this medicine
because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or asthma nurse.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor, pharmacist or asthma nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
1. What Fliveo Inhaler is and what it is used for
2. What you need to know before you use Fliveo Inhaler
3. How to use Fliveo Inhaler
4. Possible side effects
5. How to store Fliveo Inhaler
6. Contents of the pack and other information
1. WHAT FLIVEO INHALER IS AND WHAT IT IS USED FOR
Fliveo Inhaler contains the active substance fluticasone propionate. Fluticasone is a corticosteroid (often just called steroid). It has an anti-inflammatory action which reduces asthma-related swelling and irritation in the lungs. Only a very small dose of steroid is needed because it is inhaled straight into your lungs.
Your doctor has prescribed this medicine to help prevent breathing problems such as asthma. Fluticasone propionate is sometimes called a ‘preventer’.
Fliveo Inhaler is not used to treat Chronic Obstructive Pulmonary Disease (COPD).
You must use Fliveo Inhaler every day as directed by your doctor, even if you are not currently experiencing any breathing problems. This will make sure that it works properly in controlling your asthma. It takes 4-7 days for this medicine to work fully.
Fliveo Inhaler helps to prevent breathlessness and wheezing. It does not relieve sudden attacks of breathlessness or wheezing. If this happens you need to use a rapid-acting ‘reliever’ (‘rescue’) inhaler, such as salbutamol. You should have your rapid-acting ‘rescue’ inhaler with you at all times.
Fliveo Inhaler 50 micrograms is used to treat asthma in adults and children and adolescents aged 4 to 18 years. Fliveo Inhaler 50 micrograms is not recommended for children less than 4 years of age.
Fliveo Inhaler 125 micrograms and Fliveo Inhaler 250 micrograms are used to treat asthma in adults and adolescents over 16 years of age. Fliveo Inhaler 125 micrograms and Fliveo Inhaler 250 micrograms are not recommended for adolescents and children 16 years of age and younger.
2. WHAT YOU NEED TO KNOW BEFORE YOU USE FLIVEO INHALER
Do not use Fliveo Inhaler:
• If you are allergic to fluticasone propionate or the other ingredient in this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor, pharmacist or asthma nurse before using Fliveo Inhaler if you have ever had:
• Tuberculosis (TB).
• Diabetes mellitus (because fluticasone may increase your blood sugar level).
• Thinning of the bones.
• Eye problems (cataract and glaucoma).
• Growth problems (in children and adolescents).
• Cushing’s Syndrome (associated with upper body weight gain, rounding of the face, thinning of the skin and bones, depression, and various non-specific effects such as headache and fatigue).
• Adrenal suppression or an adrenal crisis.
• Treatment with steroid tablets/injections or high dose inhalers for a long period of time.
If you are not sure if any of the above applies to you, talk to your doctor, pharmacist or asthma nurse before using Fliveo Inhaler.
If you develop a chest (lung) infection during treatment (e.g. viral, fungal, or bacterial), make sure that you tell your doctor, or nurse, that you are taking this medicine and have asthma.
If you use high doses of Fliveo inhaler for a long period of time, it may affect the way your body produces its own steroid hormones. This can lead to symptoms of increased steroid hormones in the body (a condition known as Cushing’s syndrome), or reduce the amount of steroid hormones produced by the adrenal gland (adrenal suppression). Other effects include thinning of the bones, eye problems (such as cataracts or glaucoma), possible behaviour changes (for example depression or aggression) and slowing of growth in children and adolescents. For more information on these possible side effects, see ‘If you stop using Fliveo Inhaler’ (in Section 3) and ‘Possible side effects’ (Section 4).
Children and adolescents
Fliveo Inhaler should not be used in children less than 4 years of age.
Fliveo Inhaler 50 micrograms can be used to treat asthma in children and adolescents aged 4 to 18 years.
Fliveo Inhaler 125 micrograms and Fliveo Inhaler 250 micrograms can be used to treat asthma in adolescents over 16 years of age. Fliveo Inhaler 125 micrograms and Fliveo Inhaler 250 micrograms are not recommended for use in adolescents and children 16 years of age and younger.
Other medicines and Fliveo Inhaler
Tell your doctor, pharmacist or asthma nurse if you are taking, have recently taken or might take any other medicines, including medicines used for asthma and other inhalers and any non-prescription medicines. This is because Fliveo Inhaler may not be suitable to be taken with some other medicines as some medicines may increase the amount of fluticasone propionate in your body and this can increase the risk of you developing side effects or may make side effects worse. Tell your doctor if you are taking any of the following medicines before starting to use Fliveo Inhaler:
• Medicines used to treat viral infections such as ritonavir and other protease inhibitors.
• Antibiotics such as erythromycin or clarithromycin (called macrolide antibiotics).
• Antifungal tablets such as ketoconazole or itraconazole.
• Steroid tablets or steroid injections, even if you have just finished taking these. Your doctor might have given you a steroid warning card, as there is a possibility of impaired adrenal function, especially at times when your asthma is worse, or you have a chest infection or another illness, after a serious accident or if you have surgery. Your doctor may decide to give you extra steroids during this time.
If you are not sure if any of the above applies to you, talk to your doctor or pharmacist before using Fliveo Inhaler.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you might be pregnant or are planning to have a baby, ask your doctor, pharmacist or asthma nurse for advice before using this medicine.
Driving and using machines
Fliveo Inhaler is unlikely to affect your ability to drive or use machines.
3. HOW TO USE FLIVEO INHALER
Fliveo Inhaler is available in three different strengths. Your doctor will have decided which strength you need. Always use this medicine exactly as your doctor, pharmacist or asthma nurse has told you to, or as described in this leaflet. Check with your doctor, pharmacist or asthma nurse if you are not sure.
• Use Fliveo Inhaler every day until your doctor advises you to stop. Do not take more than the dose your doctor or asthma nurse has told you to take. If you are not sure how much Fliveo Inhaler you should be taking, check with your doctor, pharmacist or asthma nurse.
• Do not stop taking Fliveo Inhaler or reduce the dose that you take without talking to your doctor first.
• Fliveo Inhaler should be inhaled through the mouth into the lungs. Your doctor will decide what dose you need to take. The starting dose needs to be appropriate for the severity of your disease. Your doctor will work with you to decrease your dose to the lowest dose that effectively controls your asthma. Your doctor will want to check your symptoms regularly and do some lung function tests.
Dosage
Adults and adolescents over 16 years of age
If you have mild asthma, the recommended starting dose of Fliveo Inhaler is 100 micrograms (two puffs of Fliveo Inhaler 50 micrograms) twice daily.
If you have moderate or severe asthma, the recommended starting dose of Fliveo Inhaler is 250 micrograms (two puffs of Fliveo Inhaler 125 micrograms or one puff of Fliveo Inhaler 250 micrograms) to 500 micrograms (four puffs of Fliveo Inhaler 125 micrograms or two puffs of Fliveo Inhaler 250 micrograms) twice daily.
In some cases, your doctor or asthma specialist may prescribe a higher dose. The maximum dose is 1,000 micrograms (eight puffs of Fliveo Inhaler 125 micrograms or four puffs of Fliveo Inhaler 250 micrograms) twice daily.
Children 4 to 12 years of age and adolescents 13 to 16 years of age
Only Fliveo Inhaler 50 micrograms can be used in children 4 to 12 years of age and adolescents 13 to 16 years of age.
Fliveo Inhaler 125 micrograms and Fliveo Inhaler 250 micrograms are NOT recommended for use by children 4 to 12 years of age and adolescents 13 to 16 years of age.
The recommended starting dose of Fliveo Inhaler is 50 micrograms (one puff of Fliveo Inhaler 50 micrograms) to 100 micrograms (two puffs of Fliveo Inhaler 50 micrograms) twice daily.
In some cases, your doctor may prescribe a higher dose. The maximum dose in children 4 to 12 years of age and adolescents 13 to 16 years of age is 200 micrograms (four puffs of Fliveo Inhaler 50 micrograms) twice daily.
Whilst your child is taking this medicine, your doctor will also check your child’s height regularly.
Children under 4 years
Fliveo Inhaler is not recommended for children less than 4 years of age.
Please make sure you know how many actuations (puffs) you need to take and how and when to take them. This information should be on the pharmacist’s label on the carton in which you received your inhaler. If it is not, or you are not sure, ask your doctor, pharmacist or asthma nurse.
If your asthma or breathing gets worse, tell your doctor straight away.
You may find that you feel more wheezy, your chest feels tight more often or you may need to use more of your rapid-acting ‘reliever’ medicine or you may feel that your medicine is working less well than usual. If any of these happen, you should continue to take Fliveo Inhaler but do not increase the number of puffs that you take unless advised by your doctor. Talk to your doctor immediately as your chest condition may be getting worse and you could become seriously ill. You may need additional treatment. If required, your doctor may recommend that you use more of this medicine or may prescribe corticosteroids in tablet form. If you have been using high doses of an inhaled steroid for a long time, your doctor may decide to give you extra steroids, either by increasing the dose of your inhaler or by prescribing steroid tablets for you to take during stressful times, such as after a car accident or before surgery.
Also, patients who have been on high doses of steroids, including Fliveo Inhaler, for a long time, must not stop taking their medicine without talking to their doctor. Suddenly stopping treatment can make you feel unwell. For more information, see ‘if you stop using Fliveo inhaief later in the leaflet.
Instructions for use
• Your doctor, asthma nurse or pharmacist should show you how to use your inhaler. They should check how you use your inhaler from time to time. If you do not use your Fliveo Inhaler properly, or if you do not use it as prescribed, the medicine will not help your asthma as it should.
• The medicine is contained in a pressurised canister in a plastic casing with a mouthpiece.
• Fliveo Inhaler produces a fine mist, which must be inhaled through your mouth into your lungs.
Make sure that you know how to use this inhaler properly by reading this section. If you have any problems ask your doctor, pharmacist or asthma nurse.
Testing your inhaler (also known as ‘priming’ the inhaler)
1. When using your inhaler for the first time, test that it is working. Remove the mouthpiece cover by gently squeezing the sides with your thumb and forefinger and pull apart (figure A).
2. To make sure that the inhaler works, shake it well (figure B), point the mouthpiece away from you and press the canister firmly to release a puff into the air. Repeat these steps at least two times, shaking the inhaler before releasing each puff. If you have not used your inhaler for one day or more you should release two puffs of medicine into the air.
3. If the inhaler gets very cold, the metal canister should be taken out of the plastic actuator and warmed in your hands for a few minutes before use. Do not use anything else to warm it up.
Using your inhaler
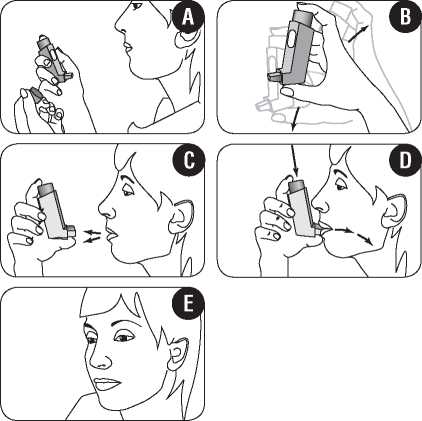
1. You should either stand up or sit upright when using your inhaler.
2. Remove the mouthpiece cover. Check that the inside and outside of the mouthpiece are clean and free of loose objects (figure A). If it needs cleaning, read the instructions under “Cleaning your inhaler” later in this leaflet.
3. Shake the inhaler well prior to use to make sure that any loose objects are removed and that the contents of the inhaler are evenly mixed (figure B).
4. Hold the inhaler upright with your thumb on the base, below the mouthpiece. Breathe out as far as is comfortable (figure C).
5. Immediately place the mouthpiece in your mouth between your teeth, and close your lips around it. Be careful not to bite the mouthpiece.
6. Breathe in slowly and deeply through your mouth. Just after starting to breathe in, press down firmly on the top of the inhaler to release a puff of medicine while still breathing in steadily and deeply (figure D).
7. Hold your breath, take the inhaler from your mouth, and take your finger from the top of the inhaler. Continue holding your breath for a few seconds, or as long as is comfortable (figure E). Then breathe out slowly.
Breathing Technique
Do not rush steps 4, 5, 6 and 7.
It is important that you breathe in as slowly as possible just before using your inhaler. You should use your inhaler whilst standing in front of a mirror for the first few times. If you see a ‘mist’ or spray coming from the top of your inhaler or the sides of your mouth, you should start again from step 3.
8. If your doctor has told you to take another puff, keep the inhaler upright, and wait about half a minute before repeating steps 3 to 7.
9. After using Fliveo Inhaler, you should rinse your mouth out with water and spit out, and/or brush your teeth. This should reduce the risk of infections in the mouth (e.g. thrush) and hoarseness of the voice.
10.Once you have finished using the inhaler, always replace the mouthpiece cover to keep out dust. Replace the cover by firmly clicking it into position.
If you find it difficult to use your inhaler and you have difficulty breathing in immediately before you press down on the top of your inhaler to release a puff of medicine while you are still breathing in or if you are taking high doses of this medicine, either your doctor or your asthma nurse may recommend that you use a spacer device, such as a Volumatic® spacer device, with your inhaler.
A spacer device should always be used by children taking this medicine. A spacer device with a face mask may be more suitable for younger children.
Only the Volumatic® spacer device should be used with Fliveo Inhaler. Other spacing devices should not be used with Fliveo Inhaler and you should not switch from one spacer device to another.
Your doctor, asthma nurse or pharmacist should show you how to use the spacer device with your inhaler and how to care for your spacer device and will answer any questions you may have.
It is important that if you or your child are using a spacer device with Fliveo Inhaler that you do not stop using it without talking to your doctor or asthma nurse first.
If you stop using a spacer device your doctor may need to change the dose of medicine required to control your asthma. Always talk to your doctor before making any changes to your asthma treatment. People with weak hands, or children, may find it easier to operate the inhaler with both hands, by putting both forefingers on the top of the inhaler, and both thumbs on the bottom below the mouthpiece. Younger children may need help from a parent or carer when using the inhaler.
Cleaning your inhaler:
Your inhaler should be cleaned at least once a week, to prevent it from blocking.
1. Remove the mouthpiece cover.
2. Do not remove the canister from the plastic casing.
3. Wipe the inside and outside of the mouthpiece and the plastic casing with a dry cloth or tissue.
Do not put the metal canister in water.
4. Discharge one actuation (puff) to waste before next use.
5. Replace the mouthpiece cover.
If you use more Fliveo Inhaler than you should It is important to use Fliveo Inhaler as stated on the pharmacist’s label or as advised by your doctor. If you accidentally take a larger dose than recommended, talk to your doctor as soon as possible. Do not change your dose without seeking medical advice.
If you have used high doses for a long period of time, you should talk to your doctor for advice. This is because high doses of this medicine may reduce the amount of steroid hormones produced by the adrenal gland (adrenal suppression).
If you forget to use Fliveo Inhaler
If you forget to use Fliveo Inhaler, take your next dose when it is due. Do not take a double dose to make up for a forgotten dose.
If you stop using Fliveo Inhaler
It is very important that you take Fliveo Inhaler every day as directed. Keep taking it until your doctor tells you to stop. Do not stop or suddenly reduce your dose of Fliveo Inhaler. This could make your asthma worse.
If you do suddenly stop taking Fliveo Inhaler or reduce your dose, particularly if you have been using high doses for a long period of time, this may reduce the amount of steroid hormones produced by the adrenal gland (adrenal suppression), which can cause side effects. These side effects may include any of the following:
• Stomach pain
• Tiredness and loss of appetite, feeling sick
• Sickness
• Weight loss
• Headache or drowsiness
• Low levels of sugar in your blood
• Low blood pressure and seizures (fits).
When your body is under stress, for example from fever, trauma (such as a car accident), infection, or surgery, adrenal suppression can get worse and you may experience any of the side effects listed above.
If you get any side effects, or you are unwell or need to go into hospital, talk to your doctor or pharmacist and tell them that you are taking this medicine. Your doctor or pharmacist may also give you a ‘steroid warning card’, which you should carry with you at all times. To prevent these side effects occurring, your doctor may prescribe extra corticosteroids in tablet form.
If you have any further questions on the use of this medicine, ask your doctor, pharmacist or asthma nurse.
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects, although not everybody gets them. To reduce the chance of side effects, your doctor will prescribe the lowest dose of this medicine to control your asthma.
If you get any of the following symptoms after using this medicine, talk to your doctor or, if serious, go to the nearest hospital emergency department immediately:
Very rare (may affect up to 1 in 10,000 people):
• Signs of an allergic reaction: you may notice itching, a rash (hives) and swelling, usually of the face, lips, mouth, tongue or throat, which may cause difficulty in swallowing or breathing, or you may suddenly feel your heart beating very fast or you feel faint and light headed (which may lead to collapse or loss of consciousness). If this happens stop using this inhaler and contact your doctor straightaway,
• Breathing difficulties or wheezing that gets worse immediately after inhaling Fliveo Inhaler. If this happens stop using this inhaler and contact your doctor straightaway. Use your rapidacting ‘reliever’ inhaler to help your breathing.
Other side effects have also been reported. If any of these trouble you, talk to your doctor, pharmacist or asthma nurse:
Very common (may affect more than 1 in 10 people):
• Thrush (sore, creamy-yellow, raised patches) in the mouth or throat, also known as oral candidiasis.
Your doctor may prescribe an antifungal medication to treat the thrush.
Common (may affect up to 1 in 10 people):
• Sore tongue, throat and hoarse voice.
• Bruising.
Problems with your mouth and throat can be reduced by doing certain things straight after taking your medicine. These are brushing your teeth, rinsing your mouth or gargling with water and spitting it out. Tell your doctor or pharmacist if you have these problems, but do not stop using this medicine unless you are told to.
Uncommon (may affect up to 1 in 100 people):
• Allergic skin rash.
Rare (may affect up to 1 in 1,000 people):
• A fungal infection of the oesophagus (the tube that connects the mouth and stomach). You may have a dry mouth and have pain or difficulty on swallowing.
Very rare (may affect up to 1 in 10,000 people):
• Sleeping problems or feeling worried (anxious), changes in behaviour such as being over-excited, restless or irritable (these effects are more likely to occur in children),
• Increased level of sugar (glucose) in your blood,
• Aching, swollen joints and muscle pain,
• Heartburn/indigestion (dyspepsia).
Using high doses of fluticasone propionate for a long period of time can cause:
• A reduction in the amount of steroid hormones produced by the adrenal gland (adrenal suppression), which if severe or if your body is stressed (fever, infection, trauma, surgery) can lead to adrenal crisis and you may become very ill. You may feel tired, you may feel sick or actually be sick, have pain in the stomach or a headache, notice weight loss or you may not want to eat, or you may have very low blood sugar which could lead to loss of consciousness or fits,
• Symptoms of increased amounts of steroid hormones in the body (Cushing’s syndrome). You may notice upper body weight gain, rounding of the face, thinning of the skin (which may bruise very easily or you may have stretch marks on the thighs, stomach, legs etc.),
• Thinning of your bones,
• Eye problems such as cataracts (clouding of the eye lens) and glaucoma (raised pressure in the eye) which may cause blurred vision,
• Slowing of growth in children and adolescents.
Your doctor will help stop these side effects from happening by making sure that you use the lowest dose of this medicine which controls your symptoms.
A serious lung infection (pneumonia) has been reported in people with chronic obstructive pulmonary disease (COPD) using fluticasone propionate. COPD is a long-term lung disease that causes shortness of breath, coughing and frequent chest infections. The term COPD includes conditions known as chronic bronchitis and emphysema. If you have difficulty breathing, have a fever, and/or have a cough which produces thick mucus which may be yellow, green or brownish or have blood in it, talk to your doctor straight away.
Not known (frequency cannot be estimated from the available data):
• Depression, restlessness, nervousness or aggression (mainly in children),
• Nose bleeds.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or asthma nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the MHRA’s Yellow Card Scheme (https://yellowcard.mhra.gov.uk). By reporting side effects, you can help provide more information on the safety of this medicine.
5. HOW TO STORE FLIVEO INHALER
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and canister after EXP. The expiry date refers to the last day of that month.
Store below 25°C. Do not refrigerate or freeze. Protect from frost and direct sunlight.
As with most inhaled medicines in pressurised canisters, the effect of this medicine may decrease when the canister is cold (see section 3 ‘Using your inhaler’).
The canister contains a pressurised liquid. Do not expose to temperatures higher than 50°C. Do not pierce, break or burn the canister, even if apparently empty.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION What Fliveo Inhaler contains
The active substance in Fliveo Inhaler is fluticasone propionate. Each metered dose (ex-valve) contains 50, 125 or 250 micrograms of fluticasone propionate. This is equivalent to a delivered dose (exactuator) of 44, 110 or 220 micrograms of fluticasone propionate respectively.
The other ingredient is the propellant: norflurane (HFA 134a).
What Fliveo Inhaler looks like and the contents of the pack
Fliveo Inhaler 50 micrograms consists of a white to off-white suspension in an aluminium canister sealed with a metering valve, inside a light orange actuator with a white dust cap.
Fliveo Inhaler 125 micrograms consists of a white to off-white suspension in an aluminium canister sealed with a metering valve, inside an orange actuator with a white dust cap.
Fliveo Inhaler 250 micrograms consists of a white to off-white suspension in an aluminium canister sealed with a metering valve, inside a dark brown actuator with a white dust cap.
Each canister contains 120 metered actuations (puffs).
Marketing Authorisation Holder
Prosonix Limited
The Magdalen Centre, The Oxford Science Park, Oxford, OX4 4GA, UK Manufacturer
Pharmaserve North West Limited, 9 Arkwright Road, Astmoor Industrial Estate, Runcorn, Cheshire, WA7 1NU, UK This medicinal product is authorised in the Member States of the EEA under following names:
Sweden: Fliveo® 50 mikrogram, 125 mikrogram,
250 mikrogram
United Kingdom: Fliveo® Inhaler 50 micrograms, 125 micrograms, 250 micrograms
This leaflet was last revised in November 2015
VOLUMATIC is a registered trade mark of Glaxo Group Limited.