Fluticasone Propionate 50 Microgram Aqueous Nasal Spray
>V
re
a
co
re
M
re
o
re
3
C
<
E
re
im
o
o
im
u
£
o
lO
re
4->
re
a
o
i_
Q.
re
c
o
M
re
u
<v
i/i
3
<V
c
o
'■?
<0
E
o
V*-
c
re
<u
<v
oi
10
u
10
Q.
Read all of this leaflet carefully before you start using this medicine because it contains important information for you
Keep this leafet. You may need to read it again.
If you have any further questions, ask your doctor or pharmacist.
This medicine has been prescribed for you only. Do not pass it on to others.lt may harm them, even if their signs of illness are the same as yours.
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Fluticasone Propionate Nasal Spray is and what it is used for
2. What you need to know before you use your Fluticasone Propionate Nasal Spray
3. Howto use Fluticasone Propionate Nasal Spray
4. Possible side effects
5. Howto store Fluticasone Propionate Nasal Spray
6. Contents ofthe packand other information
1. WHAT FLUTICASONE PROPIONATE NASAL SPRAY IS AND WHAT IT IS USED FOR
Your medicine is called Fluticasone Propionate 50 microgram Aqueous Nasal Spray (described as Fluticasone Propionate Nasal Spray' throughout this leaflet) and contains 50 micrograms ofthe active ingredient,fluticasone propionate, in each spray. Fluticasone propionate is one of a group of medicines known as corticosteroids. Fluticasone Propionate Nasal Spray has anti-inflammatory properties. When sprayed into your nose it will reduce swelling and irritation. It is used to prevent and treat seasonal allergic rhinitis (e.g. hayfever) and perennial rhinitis (e.g. blocked or runny nose and sneezing and itching caused by house dust mites or animals such as cats and dogs). It can be used by adults and children aged 4 years and older.
2. WHAT YOU NEED TO KNOW BEFORE YOU USE YOUR FLUTICASONE PROPIONATE NASAL SPRAY
Do not use Fluticasone Propionate Nasal Spray:
ifyou are allergicto fluticasone propionate or any ofthe other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talkto your doctor or pharmacist before using Fluticasone Propionate Nasal Spray: ifyou have ever had an operation to your nose ifyou are suffering or have recently suffered from an infection of the nasal airways
ifyou aresuffering or have recentlysuffered from any type of untreated infection or from tuberculosis or ocular herpes ifyou have recently been treated with injected steroids, or you have been taking oral steroids for a long time.
Fluticasone Propionate Nasal Spray will usually control seasonal allergic rhinitis (hay fever) however, ifyou are exposed to excessive amounts of pollen additional therapy may be helpful for controlling other symptoms such as itching eyes. Please consult your doctor in such an event.
Other medicines and Fluticasone Propionate Nasal Spray
Tell your doctor or pharmacist ifyou are using, have recently used or might use any other medicines.
Some medicines can interfere with Fluticasone Propionate Nasal Spray, in particular tell your doctor or pharmacist ifyou are taking:
Any medicines used in the treatment of fungal infections (e.g. ketoconazole)
Any antiviral medicine such asthose used to treat HIV infection (e.g. ritonavir)
Pregnancy and breast-feeding
Ifyou are pregnant or breast-feeding,thinkyou may be pregnant or are planning to have a baby, askyour doctor or pharmacist for advice before taking this medicine.
Driving and using machines
The medicinal product has no or negligible influence on the ability to drive and use machines.
Fluticasone Propionate Nasal Spray contains benzalkonium chloride
Fluticasone Propionate Nasal Spray contains the ingredient benzalkonium chloride solution (40 micrograms per delivered dose) which is an irritant and may cause skin reactions. If used for longer periods,the preservative benzalkonium chloride may cause nasal mucosa swelling. In case of such a reaction (persistently congested nose) medicinal products for nasal use without preservative should be used if possible. It may also cause bronchospasm.Unless such products are available another pharmaceutical form should be taken. It may also cause bronchospasm.

3. HOW TO USE FLUTICASONE PROPIONATE NASAL SPRAY
Always use this medicine exactly as your doctor or pharmacist hastold you. Check with your doctor or pharmacist if you are not sure.
The recommended dose is as follows:
Adults (including the elderly) and children aged 12 years and older:
When you first start using Fluticasone Propionate Nasal Spray you normally take two sprays in each nostril once a day, preferably in the morning.Your doctor may increase this to a maximum oftwo sprays into each nostril twice a day.
Once your symptoms are under control your doctor may reduce your dose to one spray into each nostril once a day. If the use of such a reduced dose signals a worsening in your symptoms,your dose may be increased back to the starting dose.
Children aged 4 to 11 years:
For children aged between 4-11 years old the dose is normally one spray in each nostril once a day, preferably in the morning. Your doctor may increase this to a maximum of one spray into each nostril twice a day. This product is not suitable for children under 4 years old.
Yourdoctorwill prescribethe lowest dose of Fluticasone Propionate Nasal Spray capable of effectively controlling your symptoms.
It may take a few days for this medicine to work. Do not stop using your medicine unlessyou are told to by a doctor.
You must nottake a larger dose,or use your Fluticasone Propionate Nasal Spray more often,than is prescribed by your doctor. It is important not to use more ofyour medicine than your doctor hastold you to.
If your eyes remain itchy or watery as a result of hayfever despite using this medicinetell your doctor.
He/she may be able to give you another medicine to treat your eye symptoms.
Before you use your nasal spray
Your Fluticasone Propionate Nasal Spray has a dust cap that protects the nozzle and keeps it clean - this must be taken off prior to using the spray and then replaced after use.
When your Fluticasone Propionate Nasal Spray is newthe bottle must be primed as follows:
1. Shakethe bottle gently and then remove the dust cap.
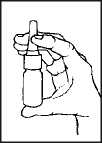
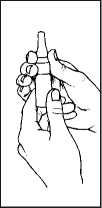
2. Hold the bottle upright withyourthumb underthe bottle and your index finger and middle finger on either side of the nozzle. Ensure that the nozzle is pointing away from you when you do this.
3. Press down with fingers in order to pump the spray.
4. Repeat steps 2 and 3 a further five times - the bottle is now primed.
Ifyou have not used your Fluticasone Propionate Nasal Spray for 7 days, prime
until a fine mist is produced.
If after attempting to primethe bottle the spray still does not workand you think
it may be blocked you may clean the spray using the following procedure:-
Cleaning your nasal spray
1. Remove the dust cap.
2. Pull the white collar upwardsto remove the nozzle.
3. Place the nozzle and dust cap in warm water and soak fora few minutes, and then rinse by placing under a running tap.
4. Shake offthe excess water and allow the nozzle and dust cap to dry in a warm (not hot) place.
5. Re-fit the nozzle.
6. 'Prime' the bottle as necessary by pumping the spray a fewtimes until a fine mist is produced.
You should clean your nasal spray at least once a weekto stop it from getting blocked up. Additional cleaning is reguired when your spray becomes blocked.
You must NEVER attemptto unblock or enlarge the spray hole with a pin or other sharp object because this will destroy the spray mechanism.
Using your nasal spray
1. Shake the bottle and remove the dust cap.
2. Gentlyblowyournose.
3. Close one nostril by pressing your finger against it and place the nozzle ofthe spray in your other nostril.
Tilt your head forward slightly so that the bottle is kept upright.
|
WIPURN |
210415-XXXX-PIL-04 | |
|
APPROVED URN |
N/A | |
|
Job |
Fluticasone PIL - Side 1 | |
|
Size |
149 x 298mm | |
|
Date |
21 April 2015 | |
|
BOH Approval Date | ||
|
Saved as |
14106/04 PIL Side 1.ai Ml | |
|
Prints |
1 Colour - Black |

4. Breathe in slowly through your open nostril and, at the same time, press the collar of the nozzle down firmly with your fingers to squirt a spray of fine mist into your nostril.
5. Breathe out through your mouth. Repeat step 4 to take a second spray into the same nostril.
6. Remove the nozzle from this nostril and breathe out through your mouth.
7. Repeat steps 3 to 6 with your other nostril.
After using your nasal spray
Wipe the nozzle carefully with a clean tissue and put the dust cap back on.
If you use more Fluticasone Propionate Nasal Spray than you should
It is important that you take your dose as stated on the pharmacist's label or as advised by your doctor. You should use only as much as your doctor recommends; using more or less may make your symptoms worse.
If you accidentally use more Fluticasone Propionate Nasal Spray than you were told to use please inform your doctor.
Take this leaflet, and your Fluticasone Propionate Nasal Spray, to show the doctor.
If you forget to use Fluticasone Propionate Nasal Spray
Do not take a double dose to make up for a forgotten dose. If you forget to take a dose at the right time, take it as soon as you remember. If it is almost time to take the next dose, wait until then and then carry on as before.
If you stop using Fluticasone Propionate Nasal Spray
Your nose symptoms may only start to improve after you have been using your medicine for a few days - therefore it is very important that you use your medicine regularly as prescribed, and that you keep using your medicine unless your doctor tells you to stop, even if you feel better.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist
4. POSSIBLE SIDE EFFECTS
Like all medicines, this medicine can cause side effects,although not everybody gets them.
If you are using high doses of Fluticasone Propionate Nasal Spray you may require extra steroids in times of extreme stress, or during admission to hospital after a serious accident or injury, or before a surgical operation.
Treatment with nasal corticosteroids may affect the production of steroids in the body. The likelihood of such an incidence is increased by use of a high dose over a long period of time. Your doctor will help prevent this happening by prescribing the lowest dose of steroid capable of adequately controlling your symptoms.
Some side effects are more serious than others and if you should experience any of the following events you should discontinue taking Fluticasone Propionate Nasal Spray and consult with your doctoras soon as possible:-
Side effects occurring very commonly (probably affecting more than 1 in 10 patients taking the Fluticasone Propionate Nasal Spray) epistaxis (nose bleeds).
Side effects occurring commonly (probably affecting up to 1 in 10 patients taking the Fluticasone Propionate Nasal Spray) headache
unpleasant taste in the mouth or an unpleasant smell in the nose dryness and irritation of the throat and nasal passages and sneezing.
Side effects occurring rarely (probably affecting fewer than 1 in every 1,000 patients taking Fluticasone Propionate Nasal Spray) severe allergic reaction giving rise to the sudden onset of a rash, swelling (usually of the tongue,face or lips) or difficulty in breathing bronchospasm (the narrowing of the airways in the lungs).
Side effects occurring very rarely (probably affecting fewer than 1 in every 10,000 patients taking the Fluticasone Propionate Nasal Spray) glaucoma (raised pressure in the eye) and cataracts (clouding of the lens in the eye) have been reported following prolonged treatment
perforation of the nasal septum (the dividing partition in the nose) and ulceration to the nose's mucus membranes - although these usually impact on patients who have had previous surgery to the nose.
Additional side effects in children and adolescents
Children may grow more slowly than others, and therefore children receiving treatment with nasal corticosteroids over a long period of time will have their height checked regularly by their doctor.Your doctor will help prevent this happening by prescribing the lowest dose of steroid capable of adequately controlling the symptoms.
Reporting of side effects
If you get side effects, talkto your doctor or pharmacist.This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via the Yellow Card Scheme at: www.mhra.qov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. HOWTO STORE FLUTICASONE PROPIONATE NASAL SPRAY
Keep out of the sight and reach of children.
Do not use Fluticasone Propionate Nasal Spray after the expiry date stated on the bottle and carton. The expiry date refers to the last day of that month.
Do not store above 25°C.
Discard after 3 months after first use.
Do not use if any signs of deterioration are seen.
Medicines should not be disposed of via wastewater or household waste. Askyour pharmacist how to dispose of medicines you no longer require. These measures will help protect the environment.
6. CONTENTS OF THE PACK AND OTHER INFORMATION
What Fluticasone Propionate Nasal Spray contains
The active substance is fluticasone propionate. Each metered spray contains 50 micrograms of fluticasone propionate.
The other ingredients are glucose anhydrous, microcrystalline cellulose, carmellose sodium, phenylethyl alcohol, benzalkonium chloride, polysorbate 80, and purified water.
What Fluticasone Propionate Nasal Spray looks like and contents of the pack
Fluticasone Propionate Nasal Spray consists of a white, opaque suspension contained within an amber glass multi-dose bottle fitted with an atomising metering pump to create the spray. Each bottle of Fluticasone Propionate Nasal Spray contains suspension capable of delivering 150 sprays.
PL holder
Manx Pharma Ltdjaylor Group House,Wedgnock Lane,Warwick,
CV34 5YA.UK.
PL 15833/0063 [Ml
Procured from within the El).
Manufacturer
Teva Czech Industries s r o, Ostravaskd 29,747 70 Opava-Kom6rov,
Czech Republic
To request a copy of this leaflet in large print, audio or Braille please call 01926482511.
This leaflet was last revised in April 2015
3*
n
ST
IQ
(D
n
(D
01
O
fi>
U)
O
3
CD
■o
o
■o
fit
«-h
to
Ul
o
3
n
O
(Q
3
3
>
.a
c
to
o
c
V)
at
w
£L
v>
■a
3
V!
WIP URN: 210415-XXXX-PIL-04
|
WIP URN |
y |
210415-XXXX-PIL-04 |
|
APPROVED URN |
N/A | |
|
Job |
Fluticasone PIL - Side 2 | |
|
Size |
149 x 298mm | |
|
Date |
21 April 2015 | |
|
BOH Approval Date | ||
|
Saved as |
14106/04 PIL Side 2.ai M1 | |
|
Prints |
1 Colour - Black |