Fluticasone Propionate 500 Micrograms Dry Powder Inhaler
Package Leaflet: Information for the user
Flixotide® 100 micrograms Accuhaler®/
Fluticasone propionate
100 micrograms Dry Powder Inhaler
Flixotide® 250 micrograms Accuhaler®/
Fluticasone propionate
250 micrograms Dry Powder Inhaler
Flixotide® 500 micrograms Accuhaler®/
Fluticasone propionate
500 micrograms Dry Powder Inhaler
(fluticasone propionate)
This product is available as any of the above names but will be referred to as Flixotide throughout the remainder of this leaflet. Please note this leaflet also contains information about other strengths (Flixotide 50 micrograms Accuhaler).
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, nurse or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others.
It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor, nurse or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1 What Flixotide is and what it is used for
2 What you need to know before you use Flixotide
3 How to use Flixotide
4 Possible side effects
5 How to store Flixotide
6 Contents of the pack and other information
1 What Flixotide is and what it is used for
Flixotide Accuhaler is a plastic inhaler device containing a foil strip with 28 or 60 blisters. Each blister contains 50, 100, 250 or 500 micrograms of the active ingredient fluticasone propionate.
Fluticasone propionate belongs to a group of medicines called corticosteroids (often just called steroids). A very small dose of steroid is needed when it is inhaled. This is because it is inhaled straight to your lungs.
Flixotide works by reducing swelling and irritation in the lungs. They have what is called an ‘anti-inflammatory action’.
Flixotide helps to prevent asthma attacks in people who need regular treatment. This is why they are sometimes called ‘preventers’. They need to be used regularly, every day.
Flixotide will not help treat sudden asthma attacks where you feel breathless.
• A different medicine is used for treating sudden attacks (called a ‘reliever’).
• If you have more than one medicine, be careful not to confuse them.
2 What you need to know before you use Flixotide
Do not use Flixotide:
• If you are allergic to fluticasone propionate or any of the other ingredients of this medicine (listed in Section 6).
Do not use Flixotide if any of the above applies to you. If you are not sure, talk to your doctor, nurse or pharmacist before using Flixotide.
Warnings and precautions
Talk to your doctor, nurse or pharmacist before taking Flixotide if:
• you have ever been treated for tuberculosis (TB)
• you are using Flixotide at the same time as taking steroid tablets. Also if you have just finished taking steroid tablets. In both cases, you should carry a steroid warning card until your doctor tells you not to carry one
If you are not sure if any of the above applies to you, talk to your doctor or pharmacist before using Flixotide.
Other medicines and Flixotide
Tell your doctor, nurse or pharmacist if you are taking, have recently taken or might take any other medicines, including medicines obtained without a prescription. This includes herbal medicines. Remember to take this medicine with you if you have to go into hospital.
In particular tell your doctor or pharmacist if you are taking any of the following:
• a type of antiviral medicine known as a ‘protease inhibitor’ (such as ritonavir)
• medicines used to treat fungal infections (such as ketoconazole)
• If you are not sure if any of the above applies to you, talk to your doctor or pharmacist before using Flixotide.
Using Flixotide with food and drink
You can use Flixotide at any time of day, with or without food.
Pregnancy and breast-feeding
If you are pregnant or are breast-feeding, think you may be pregnant or planning to have a baby, ask your doctor for advice before taking this medicine.
Driving and using machines
Flixotide is not likely to affect you being able to drive or use any tools or machines.
Flixotide contains lactose
Flixotide contains lactose (a type of sugar). If you have been told by your doctor that you cannot tolerate or digest some sugars (have an intolerance to some sugars), talk to your doctor before using this medicine.
3 How to use Flixotide
Flixotide comes in four different strengths. Your doctor will have decided which strength you need. Always use this medicine exactly as your doctor has told you. Check with your doctor, nurse or pharmacist if you are not sure.
Using this medicine
The medicine in Flixotide should be inhaled using a special kind of inhaler called an Accuhaler.
• Make sure that you have one and can use it properly
• Instructions on how to use the inhaler are given as a step-by-step guide
• You should be able to taste the powder on your tongue if you have taken it correctly
• It takes a few days for this medicine to work and it is very important that you use it regularly
Adults and Children over 16 years of age
Mild asthma
• The usual starting dose is 100 micrograms twice a day Moderate to severe asthma
• The usual starting dose is 250 to 500 micrograms twice a day
• The most taken should be 1000 micrograms twice a day
Children (4 to 16 years of age)
• The usual starting dose is 50 micrograms twice a day
• The most taken should be 200 micrograms twice a day
Flixotide Accuhaler 250 micrograms and Flixotide Accuhaler 500 micrograms are not recommended for children 16 years and under.
It is recommended that children being treated with steroids, including Flixotide Accuhaler have their height checked regularly by their doctor. Your doctor may give you a Flixotide Accuhaler of a higher strength if your dose is increased.
If you are using high doses of an inhaled steroid for a long time you may sometimes need extra steroids for example during stressful circumstances such as a road traffic accident or before an operation. Your doctor may decide to give you extra steroid medicines during this time.
Patients who have been on high doses of steroids, including Flixotide Accuhaler for a long time, must not stop taking their medicine suddenly without talking to their doctor. Suddenly stopping treatment can make you feel unwell and may cause symptoms such as vomiting, drowsiness, nausea, headache, tiredness, loss of appetite, low blood sugar level and fitting.
Instructions for use
• Your doctor, nurse or pharmacist should show you how to use your inhaler. They should check how you use it from time to time. Not using the Flixotide Accuhaler properly or as prescribed may mean that it will not help your asthma as it should.
• The Accuhaler is provided in a sealed foil wrapper. The wrapper provides protection from moisture and should only be opened when you are ready to use it for the first time. Once opened the foil wrapper should be discarded.
• The Accuhaler device holds blisters containing Flixotide as a powder.
• There is a counter on top of the Accuhaler which tells you how many doses are left. It counts down to 0. The numbers 5 to 0 will appear in red to warn you when there are only a few doses left. Once the counter shows 0, your inhaler is empty.
Do not use your inhaler more often than the doctor told you to. Tell your doctor if your medicine does not seem to be working as well as usual, as your chest problem may be getting worse and you may need a different medicine.
Your doctor may have told you to take more than this as an emergency treatment if your wheezing or breathing gets very bad. It is very important that you keep to your doctor’s instructions as to how many blisters to take and how often to use your inhaler.
Using your inhaler
1 Inside the carton, your Accuhaler is provided in a sealed foil wrapper. To open this wrapper, tear along the jagged edge, then remove the Accuhaler, and throw the wrapper away.
If you have trouble tearing the foil, do not use scissors or any other sharp objects as you may harm yourself or the Accuhaler. Ask someone to help you.
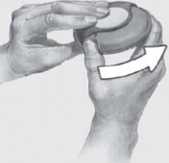
2 To open your Accuhaler, hold the outer case in one hand and put the thumb of your other hand on the thumbgrip.
Push your thumb away from you as far as it will go. You will hear a click.
This will open a small hole in the mouthpiece.
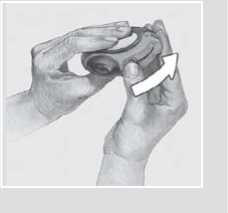
3 Hold your Accuhaler with the mouthpiece towards you. You can hold it in either your right or left hand. Slide the lever away from you as far as it will go. You will hear a click.
This places a dose of your medicine in the mouthpiece.
Every time the lever is pulled back a blister is opened inside and the powder made ready for you to inhale. Do not play with the lever as this opens the blisters and wastes medicine.
4 Hold the Accuhaler away from your mouth, breathe out as far as is comfortable. Do not breathe into your Accuhaler. Do not breathe in again yet.
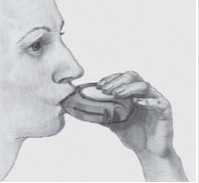
5 Put the mouthpiece to your lips; breathe in steadily and deeply through the Accuhaler with your mouth, not through your nose. Remove the Accuhaler from your mouth.
Hold your breath for about 10 seconds or for as long as is comfortable. Breathe out slowly.
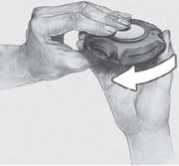
6 To close the Accuhaler, slide the thumbgrip back towards you, as far as it will go.
You will hear a click. The lever will return to its original position and is reset.
Your Accuhaler is now ready for you to use again.
Afterwards, rinse your mouth with water and spit it out.
Cleaning your Accuhaler
Wipe the mouthpiece of the Accuhaler with a dry tissue to clean it.
If you use more Flixotide than you should
If you use more than you should, talk to your doctor as soon as possible.
It is important that you take your dose as stated on the pharmacist’s label or as advised by your doctor. You should not increase or decrease your dose without seeking medical advice.
If you forget to use Flixotide
• Take the next dose when it is due.
• Do not take a double dose to make up for the forgotten dose.
If you stop using Flixotide
• Do not stop treatment even if you feel better unless told to do so by your doctor.
If you have any further questions on the use of this medicine, ask your doctor, nurse or pharmacist.
4 Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you notice any of the following serious side effects, stop using this medicine and talk to your doctor straight away. You may need urgent medical treatment.
• allergic reactions (may affect up to 1 in 100 people) -the signs include skin rashes, redness, itching or weals like nettle rash or hives
• severe allergic reactions (may affect up to 1 in 10,000 people) - the signs include swelling of your face, lips, mouth, tongue or throat which may cause difficulty in swallowing or breathing, itchy rash, feeling faint and light headed and collapse
• your breathing or wheezing gets worse straight after using your inhaler
Other side effects include:
Very common (may affect more than 1 in 10 people)
• thrush in the mouth and throat
Common (may affect up to 1 in 10 people)
• sore tongue or throat
• hoarseness of voice
Problems with your mouth and throat can be reduced by doing certain things straight after inhaling your dose. These are brushing your teeth, rinsing your mouth or gargling with water and spitting it out. Tell your doctor if you have these problems with your mouth or throat, but do not stop treatment unless you are told to.
The following side effects have also been reported in patients with Chronic Obstructive Pulmonary Disease (COPD):
• Pneumonia and bronchitis (lung infection). Tell your doctor if you notice any of the following symptoms: increased sputum production, change in sputum colour, fever, chills, increased cough, increased breathing problems
• Bruising
Rare (may affect up to 1 in 1,000 people)
• thrush (candidiasis) in the oesophagus
Very rare (may affect up to 1 in 10,000 people)
• sleeping problems or feeling worried, over-excited and irritable. These effects are more likely to occur in children
• joint pains
• indigestion
• level of sugar (glucose) in your blood may be increased
• the way steroids are produced by your body may be affected when using Flixotide. This is more likely to happen if you use high doses for a long period of time. This can cause:
- children and young people to grow more slowly
- something called ‘Cushing’s syndrome’. This happens when you have too much steroid in your body and it can cause thinning of your bones and eye problems (such as cataracts and glaucoma which is high pressure in the eye)
Your doctor will help stop this happening by making sure you use the lowest dose of steroid which controls your symptoms.
Although the frequency is not known, the following side effects may also occur:
• depression, feeling restless or nervous. These effects are more likely to occur in children
• nosebleeds
Talk to your doctor as soon as possible if:
• after 7 days of using Flixotide your shortness of breath or wheezing does not get better, or gets worse
• you or your child is on high doses of inhaled steroid and become unwell with vague symptoms such as tummy ache, sickness, diarrhoea, headache or drowsiness. This can happen during an infection such as a viral infection or stomach upset. It is important that your steroid is not stopped suddenly as this could make your asthma worse and could also cause problems with the body’s hormones
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5 How to store Flixotide
• Keep out of the sight and reach of children.
• Do not store above 30°C. Store in a dry place to protect from moisture. Store the Accuhaler in the foil wrapper until you are ready to use it for the first time. Once opened, the foil wrapper should be discarded.
• If you are told to stop taking this medicine return Flixotide Accuhaler to the pharmacist for disposal.
• Do not use Flixotide after the expiry date which is stated on the carton. The expiry date refers to the last day of that month.
• Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
• If your medicine become discoloured or show signs of any deterioration, you should seek the advice of your pharmacist.
6 Contents of the pack and other information
What Flixotide contains
Flixotide is provided in a sealed foil wrapper.
Flixotide Accuhaler contains the active ingredient fluticasone propionate and the inactive ingredient lactose monohydrate which acts as the ‘carrier’. It is a two tone orange and brown colour, circular device in moulded plastic containing inhalation powder and with a dose counter indicating number of doses remaining. The blister protects the powder for inhalation from the effects of the atmosphere. Each blister contains 100, 250 or 500 micrograms of the active ingredient fluticasone
propionate. The device has a counter which tells you the number of blisters remaining. It counts down from 60 to 0. To show when the last five blisters have been reached the numbers appear in red. When the counter shows 0 your inhaler is empty and should be disposed of.
Manufacturer
Flixotide is manufactured by Glaxo Wellcome Production, Zone Industrielle 2, Rue Lavoiser 23, F-27000 Evreux, France and is procured from within the EU by the Product Licence holder: Caseview (PL) Ltd., 20 Alliance Court, Alliance Road, London W3 ORB and repackaged by OPD Laboratories Ltd, Unit 6 Colonial Way, Watford, Herts WD24 4PR.
POM
PL 13826/0553
Flixotide® 100 micrograms Accuhaler®/ Fluticasone propionate 100 micrograms Dry Powder Inhaler
PL 13826/0554
Flixotide® 250 micrograms Accuhaler® / Fluticasone propionate 250 micrograms Dry Powder Inhaler
PL 13826/0555
Flixotide® 500 micrograms Accuhaler® / Fluticasone propionate 500 micrograms Dry Powder Inhaler
Leaflet revision date (ref): 25.08.2015. Flixotide and Accuhaler are trademarks of the GSK group of companies.
To request a copy of this leaflet in Braille, large print or audio please call 01923 332 796.