Fml
PACKAGE LEAFLET: INFORMATION FOR THE USER
FML® /
FLUOROMETHOLONE 0.1% EYE DROPS SUSPENSION (fluorometholone)
Your medicine is available using one of the above names, but will be referred to as FML throughout this leaflet.
Read all of this leaflet carefully before you start using this medicine.
• Keep this leaflet. You may need to read it again.
• If you have further questions, please ask your doctor or your pharmacist.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
• If any of the side effects get serious, or if you notice any side effects not listed in this leaflet please tell your doctor or pharmacist.
In this leaflet:
1. What FML is and what it is used for
2. Before you use FML
3. How to use FML
4. Possible side effects
5. How to store FML
6. Further information
1. WHAT FML IS AND WHAT IT IS USED FOR
FML is an eye drop. It contains a steroid which is used to treat inflammation of the eye.
2. BEFORE YOU USE FML
Do not use FML
• if you are allergic (hypersensitive) to fluorometholone, benzalkonium chloride, or any of the other ingredients of FML.
• if you have a bacterial, viral, or fungal infection of the eye.
Take special care with FML
You should not use FML for more than one week unless your doctor or eye specialist advises it.
Prolonged use may cause the pressure inside your eye (intraocular pressure) to increase, which could lead to glaucoma, rare damage to the optic nerve, lack of clearness of vision, cataracts, delay in wound healing, or the development of an eye infection. The pressure in your eye will be regularly measured.
If you have or have been previously treated for herpes simplex, use FML only under close supervision of your doctor.
The use of the bottle by more than one person may spread infection.
Using other medicines
Please tell your doctor or pharmacist if you are taking, or have recently taken, any other medicines, even those obtained without a prescription.
If you are using other eye drops, leave at least 5 minutes before putting in FML. Children
The safety and effectiveness in children aged 2 years or less have not been shown.
Pregnancy and breast-feeding
Tell your doctor before you start using FML if you are pregnant or planning to become pregnant or if you are breast-feeding. Your doctor will decide if you should use FML during pregnancy or whilst breast-feeding. Normally it is not recommended for use.
Driving and using machines
FML can cause temporary blurred vision. Should this occur, wait for the blurring to clear before driving or operating machinery or taking part in any activity where this could put yourself or others at risk.
Important information about some of the ingredients of FML
FML contains benzalkonium chloride as a preservative, which is known to discolour soft contact lenses and can cause eye irritation. If you wear soft contact lenses you must remove them before using FML eye drops and wait at least 15 minutes before putting your lenses back in.
Ask your doctor or pharmacist for advice before taking any medicine.
3. HOW TO USE FML
Always use FML exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
The usual dose is 1 or 2 drops of FML in each eye that needs treatment 2 to 4 times a day, or more frequently if your doctor advises it. During the first 24 - 48 hours of treatment your doctor may advise you to apply 2 drops at one hour intervals.
Treatment should not be withdrawn too early.
Instructions for use
You must not use the bottle if the seal on the bottle neck is broken before you first use it.
Shake the bottle before use. Wash your hands before opening the bottle.
Use your eye drops in the following way:
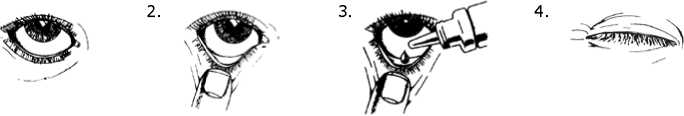
1. Tilt your head back and look at the ceiling.
2. Gently pull down the lower eyelid until there is a small pocket.
3. Turn the bottle upside down and gently squeeze it to release 1 or 2 drops into the eye.
4. Let go of the lower lid, and close your eye for 30 seconds.
5. Repeat steps 2 to 4 for the other eye, if it also needs treatment.
If a drop misses your eye, try again.
To keep the eye drops free from contamination and avoid eye injury, do not let the tip of the
bottle touch your eye or anything else. Put the cap back on and close the bottle tightly straight
after use. Wipe off any excess liquid from your cheek with a clean tissue.
If you use more FML than you should
If you use more drops of FML than you should, it is unlikely to cause you any harm. If you have placed too many drops in your eye(s), wash your eyes with clean water. Put your next dose in at the usual time.
If you drink FML by accident
If anyone drinks FML by accident, it is unlikely to cause any harm. The affected person should drink fluids to dilute.
If you forget to use FML
If you forget a dose, use FML as soon as you remember, unless it is almost time for your next dose. Then use your next dose at the usual time. Do not take a double dose to make up for a forgotten dose.
If you stop using FML
To work properly FML should be used as advised by your doctor or pharmacist.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. POSSIBLE SIDE EFFECTS
Like all medicines, FML can have side effects, although not everybody gets them. The following side effects are known to occur but their frequency can vary, as shown.
If you experience swallowing or breathing difficulties, swelling of your lips, face, throat or tongue contact your doctor or go to the hospital immediately as this could be a sign of a serious allergic reaction. The frequency of an allergic reaction is not known.
Affecting the eve
Frequency - Common faffectina up to 1 in 10 patients!:
Increased pressure inside your eye
Frequency not known:
Cataract (a loss of transparency of the lens of the eye with partial or complete loss of vision)
Eye irritation Eye inflammation Redness of the eye Itching of the eye Eye pain
A feeling that something is in your eye Difficulty in seeing clearly Swelling of the eyelid or eye Eye discharge
Excessive dilatation of the pupil Excessive tear production Ulcer(s) on the surface of the eye Small breaks in the surface of the eye Visual field defects
Secondary infections (including bacterial, fungal, and viral infections)
Affecting the body Frequency not known:
Rash, abnormal sense of taste, allergic reactions (hypersensitivity)
Other side effects reoerted with eve drcos containing ohcsohates
In very rare cases, some patients with severe damage to the clear layer at the front of the eye (the cornea) have developed cloudy patches on the cornea due to calcium build-up during treatment.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.aov.uk/vellowcard. By reporting side effects, you can help provide more information on the safety of this medicine.
5. HOW TO STORE FML
Keep out of the sight and reach of children.
Do not store above 25°C (room temperature). Do not freeze.
Throw the bottle away 28 days after opening, even if there is solution remaining.
This medicine is for your use only. It can only be prescribed by a doctor. Never give it to anyone else. It may harm them even if their symptoms are the same as yours.
Warnings
Do not use the product if the tamper-proof seal on the bottle is broken.
The product should not be used after the expiry date. (This is printed on both the label on the bottle and on the bottom of the carton that the bottle is packed in.)
If your doctor decides to stop treatment, return any leftover eye drops to the pharmacist. Only keep them if your doctor tells you to.
If the eye drops appear discoloured or show any other signs of deterioration, take them to your pharmacist who will advise you.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. FURTHER INFORMATION
What FML contains
The active ingredient in FML is: fluorometholone. Each ml contains lmg of fluorometholone.
It also contains: polyvinyl alcohol, edetate disodium, benzalkonium chloride, polysorbate 80, sodium phosphate, dibasic, heptahydrate, sodium phosphate, monobasic, monohydrate, sodium chloride, purified water, and sodium hydroxide to adjust pH.
What FML looks like and contents of the pack
FML is a sterile eye drop suspension and each bottle contains 5ml of the medicine. Manufacturer
FML is manufactured by: Allergan Pharmaceuticals (Ireland) Limited, Westport, Co. Mayo, Ireland.
Procured from within the EU and repackaged by: Doncaster Pharmaceuticals Group Ltd., Kirk Sandall, Doncaster, DN3 1QR.
Product Licence holder: Doncaster Pharmaceuticals Group Ltd., Kirk Sandall, Doncaster, DN3 1QR.
POM
PL No: 04423/0388
Leaflet revision and issue date (Ref): 26.11.15 FML® is a registered trademark of Allergan, Inc.
To listen to or request a copy of this leaflet in Braille, large print or audio please call 01302 365000 and ask for the Regulatory Department.
Please be ready to give the following information:
Product name FML / FLUOROMETHOLONE 0.1% EYE DROPS SUSPENSION
Reference number 04423/0388
2 of 2