Fortum 500 Mg Powder For Solution For Injection
The following information is intended for healthcare professionals only:
10000000140502

Fortum® 500 mg powder for solution for injection Fortum® 1 g, 2 g, 3 g powder for solution for injection or infusion
ceftazidime
3 years.
After reconstitution:
Chemical and physical in-use stability has been demonstrated for 6 days at 4°C and 9 hours at 25°C in Water for Injections or compatible fluids listed below.
From a microbiological point of view, the reconstituted solution should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2 to 8°C, unless reconstitution has taken place in controlled and validated aseptic conditions.
After dilution:
Chemical and physical in-use stability has been demonstrated for 6 days at 4°C and 9 hours at 25°C in Water for Injections or compatible fluids listed below.
From a microbiological point of view, the reconstituted and diluted solution should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2 to 8°C, unless reconstitution has taken place in controlled and validated aseptic conditions.
Special precautions for storage
Store below 25°C.

Ceftazidime at concentrations between 1 mg/ml and 40 mg/ml is compatible with:
• sodium chloride 9 mg/ml (0.9%) solution for injection
• M/6 sodium lactate injection .
• compound sodium lactate injection (Hartmann's solution) . .
• 5% dextrose injection
• 0.225% sodium chloride and 5% dextrose injection
• 0.45% sodium chloride and 5% dextrose injection
• 0.9% sodium chloride and 5% dextrose injection
• 0.18% sodium chloride and 4% dextrose injection
• 10% dextrose Injection
• Dextran 40 injection 10% in 0.9% sodium chloride injection
• Dextran 40 injection 10% in 5% dextrose Injection
• Dextran 70 injection 6% in 0.9% sodium chloride injection
• Dextran 70 injection 6% in 5% dextrose injection.
Ceftazidime at concentrations between 0.05 mg/ml and 0.25 mg/ml is compatible with Intra-peritoneal Dialysis Fluid (Lactate).
Ceftazidime at concentrations detailed in Table 1 may be constituted for intramuscular use with 0.5% or 1% Lidocaine Hydrochloride Injection.
The contents of a 500 mg vial of ceftazidime for injection, constituted with 1.5 ml water for injections, may be added to metronidazole injection (500 mg in 100 ml) and both retain their activity.
500 mg powder for solution for injection, 1 g, 2 g, 3 g powder for solution for injection or infusion.:
Preparation of solution for bolus injection:
Keep vials in the outer carton to protect from light.
Special precautions for disposal and other handling
All sizes of vials of Fortum are supplied under reduced pressure. As the product dissolves, carbon dioxide is released and a positive pressure develops. Small bubbles of carbon dioxide in the constituted solution may be ignored.
Instructions for constitution
See Table 1 and Table 2 for addition volumes and solution concentrations, which may be useful when fractional doses are required.
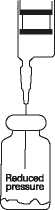
1. Insert the syringe needle through the vial closure and inject the recommended volume of diluent. The vacuum may assist entry of the diluent. Remove the syringe needle.
5^
pressure

. J
2. Shake to dissolve: carbon dioxide is released and a clear solution will be obtained in about 1 to 2 minutes.
Table 1: Powder for Solution for Injection
|
Presentation |
Amount of diluent to be added (ml) Approximate concentration (mg/ml) | ||
|
500 mg | |||
|
Intramuscular Intravenous bolus |
1.5 ml 5 ml |
260 90 | |
|
1g_ | |||
|
Intramuscular Intravenous bolus |
3 ml 10 ml |
260 90 | |
|
2g_ | |||
|
Intravenous bolus |
10 ml |
170 | |
|
3o | |||
|
Intravenous bolus |
15 ml |
170 | |
Note:
• The resulting volume of the solution of ceftazidime in reconstitution medium is increased due to the displacement factor of the drug product resulting in the listed concentrations in mg/ml presented in the above table.
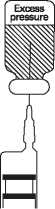
3. Invert the vial. With the syringe plunger fully depressed, insert the needle through the vial closure and withdraw the total volume of solution into the syringe (the pressure in the vial may aid withdrawal). Ensure that the needle remains within the solution and does not enter the head space. The withdrawn solution may contain small bubbles of carbon dioxide; they may be disregarded.
These solutions may be given directly into the vein or introduced into the tubing of a giving set if the patient is receiving parenteral fluids. Ceftazidime is compatible with the intravenous fluids listed above. 1 g, 2 g, 3 g powder for solution for injection or infusion.:
Preparation of solutions for iv infusion from ceftazidime injection in standard vial presentation (mini-bag or burette-type set):
Table 2: Powder for Solution for Infusion
|
Presentation |
Amount of diluent to be added (ml) |
Approximate concentration (mg/ml) | |
|
1 g | |||
|
Intravenous infusion |
50 ml* |
20 | |
|
2 g | |||
|
Intravenous infusion |
50 ml* |
40 | |
|
3 g | |||
|
Intravenous infusion |
75 ml* |
40 | |
* Addition should be in two stages.
Prepare using a total of 50 ml (for 1 g and 2 g vials) and 75 ml (for 3 g vials) of compatible diluents
(listed above), added in TWO stages as below.
1. Introduce the syringe needle through the vial closure and inject 10 ml of diluent for the 1 g and 2 g vials, and 15 ml for the 3 g vial.
2. Withdraw the needle and shake the vial to give a clear solution.
3. Do not insert a gas relief needle until the product has dissolved. Insert a gas relief needle through the vial closure to relieve the internal pressure.
4. Transfer the reconstituted solution to final delivery vehicle (e.g. mini-bag or burette-type set) making up a total volume of at least 50 ml (75 ml for the 3 g vial), and administer by intravenous infusion over 15 to 30 min.
Note:
• The resulting volume of the solution of ceftazidime in reconstitution medium is increased due to the displacement factor of the drug product resulting in the listed concentrations in mg/ml presented in the above table.
Solutions range in colour from light yellow to amber depending on concentration, diluent and storage conditions used. Within the stated recommendations, product potency is not adversely affected by such colour variations.
Note: To preserve product sterility, it is important that the gas relief needle is not inserted through the vial closure before the product has dissolved.
Any residual antibiotic solution should be discarded.
For single use only.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements. This leaflet was last revised in May 2016.
The information in this leaflet applies only to Fortum for Injection.
Fortum is a registered trade mark of the GSK group of companies.
© 2016 GSK group of companies. All rights reserved.
---------------------------------------------------------------------------------------------------------------------------Xg
Package Leaflet: Information for the User
Fortum® 500 mg powder for solution for injection Fortum® 1 g, 2 g, 3 g powder for solution for injection or infusion
ceftazidime
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or nurse.
• If you get any side effects talk to your doctor or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1 What Fortum is and what it is used for
2 What you need to know before you are given Fortum
3 How Fortum is given
4 Possible side effects
5 How to store Fortum
6 Contents of the pack and other information
• a type of antibiotic called aminoglycosides e.g. gentamicin, tobramycin
• water tablets called furosemide
Tell your doctor if this applies to you. Pregnancy, breast-feeding and fertility Ask your doctor for advice before you are given Fortum:
• If you are pregnant, think you might be pregnant or are planning to become pregnant
• If you are breast-feeding
Your doctor will consider the benefit of treating
you with Fortum against the risk to your baby.
Driving and using machines
Fortum can cause side effects that affect your
ability to drive, such as dizziness. Don't drive
or use machines unless you are sure you're not
affected.
Fortum contains sodium
You need to take this into account if you are on a controlled sodium diet.
Fortum is an antibiotic used in adults and children (including newborn babies). It works by killing bacteria that cause infections. It belongs to a group of medicines called cephalosporins.
Fortum is used to treat severe bacterial infections of:
• the lungs or chest
• the lungs and bronchi in patients suffering from cystic fibrosis
• the brain (meningitis)
• the ear
• the urinary tract
• the skin and soft tissues
• the abdomen and abdominal wall (peritonitis)
• the bones and joints.
Fortum can also be used:
• to prevent infections during prostate surgery in men
• to treat patients with low white blood cell counts (neutropenia) who have a fever due to a bacterial infection.
You must not be given Fortum:
• if you are allergic to ceftazidime or any of
the other ingredients of this medicine (listed in section 6).
• if you have had a severe allergic reaction to any other antibiotic (penicillins, monobactams and carbapenems) as you may also be allergic to Fortum.
^ Tell your doctor before you start on Fortum if you think that this applies to you. You must not be given Fortum.
Take special care with Fortum
You must look out for certain symptoms such as allergic reactions, nervous system disorders and gastrointestinal disorders such as diarrhoea while you are being given Fortum. This will reduce the risk of possible problems. See ('Conditions you need to look out for') in section 4. If you have had an allergic reaction to other antibiotics you may also be allergic to Fortum.
If you need a blood or urine test Fortum can affect the results of urine tests for sugar and a blood test known as the Coombs test. If you are having tests:
^ Tell the person taking the sample that you have been given Fortum.
Other medicines and Fortum Tell your doctor if you are taking, have recently taken or might take any other medicines. This includes medicines you can obtain without a prescription.
You shouldn't be given Fortum without talking to your doctor if you are also taking:
• an antibiotic called chloramphenicol
Fortum is usually given by a doctor or nurse.
It can be given as a drip (intravenous infusion) or as an injection directly into a vein or into a muscle.
Fortum is made up by the doctor, pharmacist or nurse using water for injections or a suitable infusion fluid.
The recommended dose
The correct dose of Fortum for you will be decided by your doctor and depends on: the severity and type of infection; whether you are on any other antibiotics; your weight and age; how well your kidneys are working.
Newborn babies (0-2 months)
For every 1 kg the baby weighs, they'll be given 25 to 60 mg Fortum per day divided in two doses.
Babies (over 2 months) and children who
weigh less than 40 kg
For every 1 kg the baby or child weighs, they'll be given 100 to 150 mg of Fortum per day divided in three doses. Maximum 6 g per day.
Adults and adolescents who weigh 40 kg or more
1 to 2 g of Fortum three times daily. Maximum of 9 g per day.
Patients over 65
The daily dose should not normally exceed 3 g per day, especially if you are over 80 years of age. Patients with kidney problems You may be given a different dose to the usual dose. The doctor or nurse will decide how much Fortum you will need, depending on the severity of the kidney disease. Your doctor will check you closely and you may have more regular kidney function tests.
If you are given more Fortum than you should
If you accidentally use more than your prescribed dose, contact your doctor or nearest hospital straight away.
If you forget to use Fortum
If you miss an injection, you should have it as soon as possible. Don't take a double dose (two injections at the same time) to make up for a missed dose.
Don't stop taking Fortum Don't stop taking Fortum unless your doctor tells you to. If you have any further questions on the use of this medicine ask your doctor or nurse.
|
Fortum Strength |
Amount per vial |
|
Fortum 500 mg |
26 mg |
|
Fortum 1 g |
52 mg |
|
Fortum 2 g |
104 mg |
|
Fortum 3 g |
156 mg |
3. How Fortum is given
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Conditions you need to look out for
The following serious side effects have occurred in a small number of people but their exact frequency is unknown:
• severe allergic reaction. Signs include raised and itchy rash, swelling, sometimes of the face or mouth causing difficulty in breathing.
• Skin rash, which may blister, and looks like small targets (central dark spot surrounded by a paler area, with a dark ring around the edge).
• A widespread rash with blisters and peeling skin. (These may be signs of Stevens-Johnson syndrome or toxic epidermal necrolysis).
• Nervous system disorders: tremors, fits and, in some cases coma. These have occurred in people when the dose they are given is too high, particularly in people with kidney disease.
• There have been rare reports of severe hypersensitivity reactions with severe rash, which may be accompanied by fever, fatigue, swelling of the face or lymph glands, increase of eosinophils (type of white blood cells), effects on liver, kidney or lung (a reaction called DRESS).
^ Contact a doctor or nurse immediately if you get any of these symptoms.
Common side effects
These may affect up to 1 in 10 people:
• diarrhoea
• swelling and redness along a vein
• red raised skin rash which may be itchiness
• pain, burning, swelling or inflammation at the injection site.
^ Tell your doctor if any of these are troubling you.
Common side effects that may show up in blood tests:
• an increase in a type of white blood cell (eosinophilia)
• an increase in the number of cells that help the blood to clot
• an increase in liver enzymes.
Uncommon side effects
These may affect up to 1 in 100 people:
• inflammation of the gut which can cause pain, or diarrhoea which may contain blood
• thrush (fungal infections in the mouth or vagina)
• headache
• dizziness
• stomach ache
• feeling sick or being sick
• fever and chills.
^ Tell your doctor if you get any of these. Uncommon side effects that may show up in blood tests:
• a decrease in the number of white blood cells
• a decrease in the number of blood platelets (cells that help the blood to clot)
• an increase in the level of urea, urea nitrogen or serum creatinine in the blood.
Very rare side effects
These may affect up to 1 in 10,000 people:
• inflammation or failure of the kidneys
Other side effects
Other side effects have occurred in a small number of people but their exact frequency is unknown:
• inflammation or failure of the kidneys
• pins and needles
• unpleasant taste in the mouth
• yellowing of the whites of the eyes or skin. Other side effects that may show up in blood tests:
• red blood cells destroyed too quickly
• an increase in a certain type of white blood cells
• severe decrease in the number of white blood cells.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
Keep this medicine out of the sight and reach of
children.
• Do not use this medicine after the expiry date which is stated on the vial and carton after EXP. The expiry date refers to the last day of that month.
• Store below 25°C.
• Keep vials in the outer carton to protect from light.
• Reconstituted and diluted solution: The doctor, pharmacist or nurse will make up your medicine in Water for Injections or compatible fluids. Once made up, this medicine should be used within 6 days if stored in refrigerator (at 4°C) or within 9 hours if stored at room temperature (below 25°C).
• Do not throw away any medicines via wastewater or household waste. Your doctor or nurse will throw away any medicine that is no longer required. This will help protect the environment.
What Fortum contains
• Fortum is available in the following strengths: 3 g, 2 g, 1 g and 500 mg. The active substance is 3 g, 2 g, 1 g or 500 mg of ceftazidime (present as ceftazidime pentahydrate).
• The only other ingredient is sodium carbonate (anhydrous sterile).
• See section 2 for further important information about sodium, one of the ingredients of Fortum.
What Fortum looks like and contents of the pack
Fortum 500 mg powder for solution for injection is a sterile white to cream powder filled in glass 17 ml vial with a bromobutyl rubber plug and a flip-off type aluminium overseal.
Available in packs of 1, 5 or 10 vials.
Fortum 1 g powder for solution for injection or infusion is a sterile white to cream powder filled in glass 17 ml, 26 ml, 60 ml or 77 ml vial with a bromobutyl rubber plug and a flip-off type aluminium overseal.
Available in packs of 1, 5, 10, 50 or 100 vials. Fortum 2 g powder for solution for injection or infusion is a sterile white to cream powder filled in glass 60 ml or 77 ml vial with a bromobutyl rubber plug and a flip-off type aluminium overseal. Available in packs of 1, 5, 10, 25 or 50 vials. Fortum 3 g powder for solution for injection or infusion is a sterile white to cream powder filled in glass 127 ml vial with a bromobutyl rubber plug and a flip-off type aluminium overseal. Available as a single pack of 1 vial.
Not all pack sizes may be marketed.
Your doctor, pharmacist or nurse will make the injection or infusion up with Water for Injections or a suitable infusion fluid. When made up, Fortum varies in colour from light yellow to amber. This is perfectly normal.
Marketing Authorisation Holder and Manufacturer
Marketing Authorisation Holder:
Glaxo Operations UK Ltd., Stockley Park West, Uxbridge, Middlesex. UB11 1BT Manufacturer: GlaxoSmithKline Manufacturing S.p.A., Verona, Italy.
Other formats
To listen to or request a copy of this leaflet in Braille, large print or audio please call, free of charge:
0800 198 5000 (UK Only)
Please be ready to give the following information:
Product names Fortum for Injection 3 g, 2 g, 1 g or 500 mg
Reference numbers 00004/0292
This is a service provided by the Royal National
Institute of Blind People.
This leaflet was last revised in May 2016. Fortum is a registered trade mark of the . GSK group of companies. . .
© 2016 GSK group of companies. All rights reserved.
10000000140502