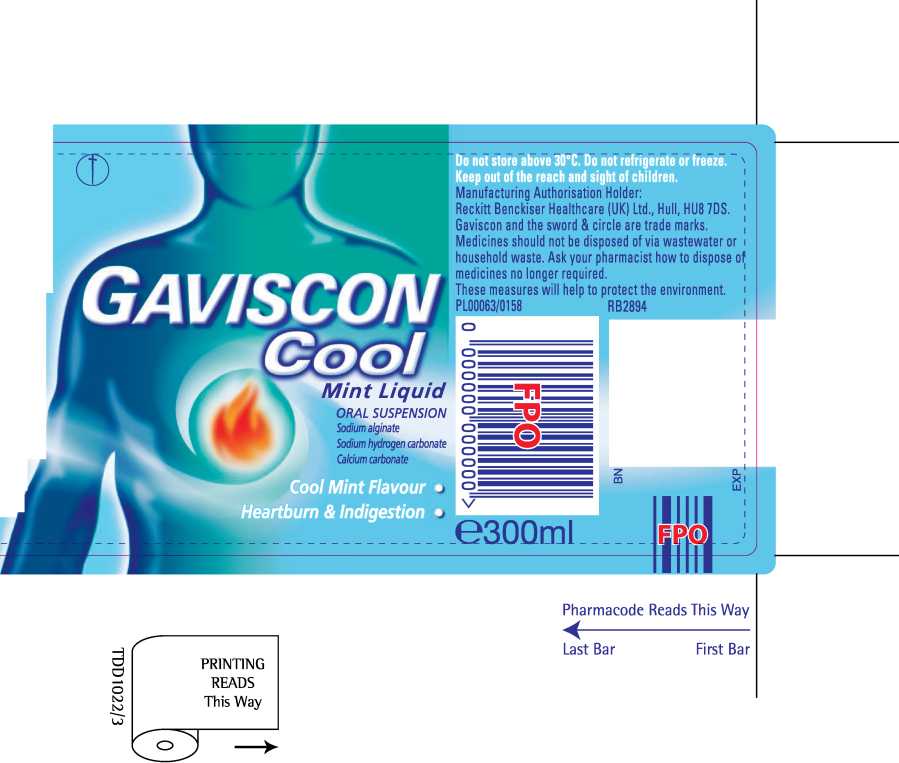
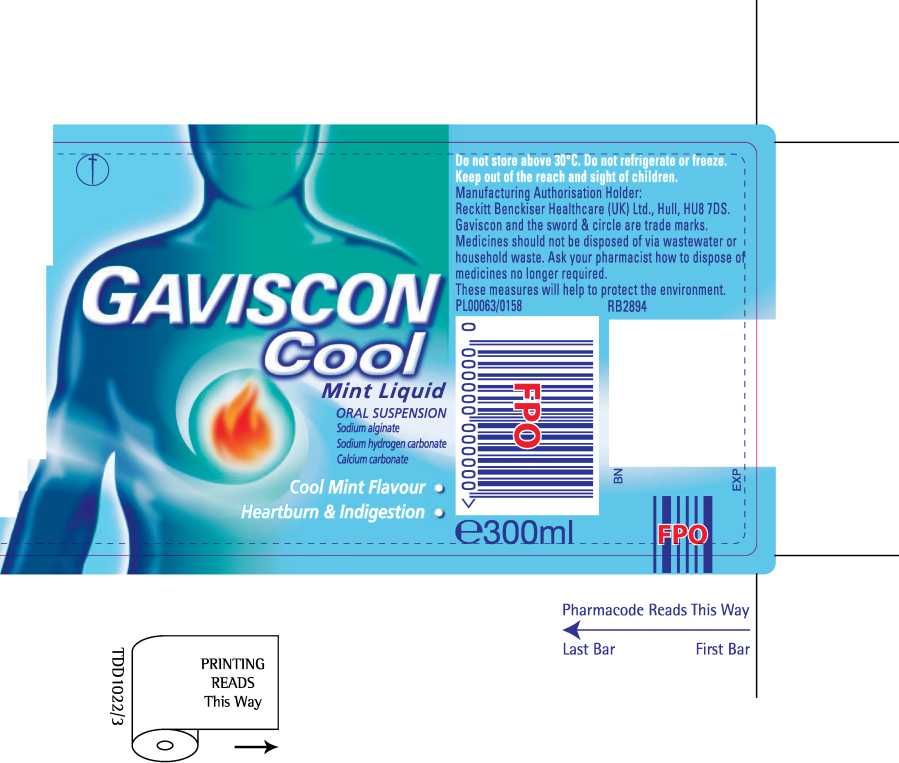
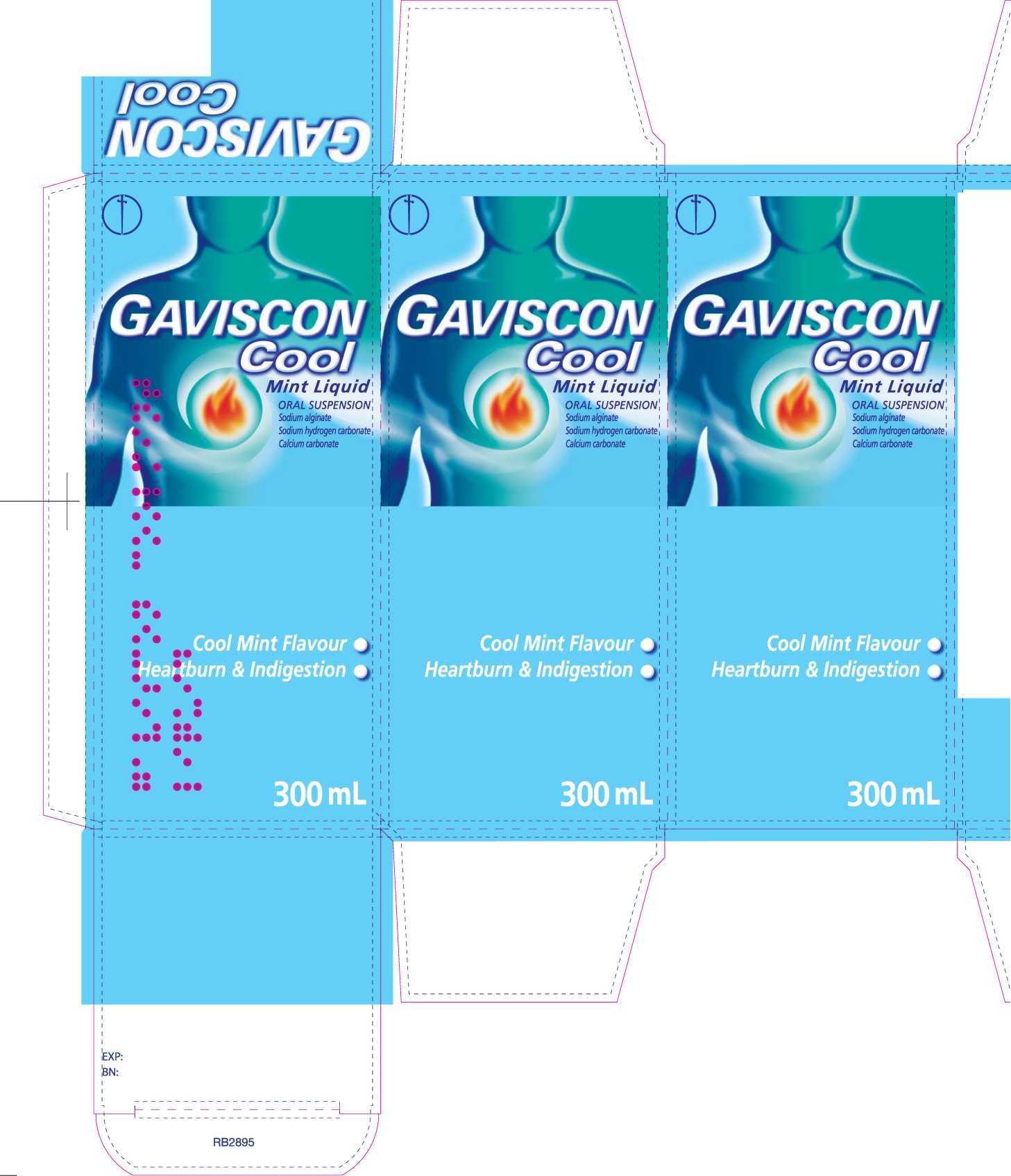
Gaviscon Cool Mint Liquid
' Gaviscon Cool MintLiquTdTmngs reliefTrom tFe pain and" discomfort of heartburn and acid indigestion, which for example, can occur after meals or during pregnancy.
The product belongs to a group of medicines called 'reflux suppressants', which form a protective layer on top of the stomach contents to prevent acid escaping from the stomach where it works into the food pipe where it hurts.
Each 10ml dose of oral suspension contains sodium alginate 500mg, sodium hydrogen carbonate 267mg and calcium carbonate 160mg as the active ingredients. Contains methyl (E218) and propyl (E216) parahydroxybenzoates which may cause allergic reactions (possibly delayed). This product does not contain sugar or gluten. You can take this product if you are pregnant or b
EGb Adults, including the and children 12 years and over: 10-20ml (two to four 5ml spoonfuls) after meals and at bedtime (up to four times a day). Children under 12 years: Should only be taken on medical advice. If symptoms persist after 7 days consult your doctor. Contains sodium and calcium.
If you have been advised to follow a diet restricted ini either of these salts, please consult your doctor before taking this product.___________
RBH Artwork
|
and Print Specification | |
|
Trident Reference No: TR361367 | |
|
Action / Version: |
c |
|
Brand: |
Gaviscon |
|
Brand Platform: |
Adult |
|
Sub Platform: |
Cool |
|
Sub Brand: |
Cool Liquid |
|
Format: |
Liquid |
|
Delivery: |
Bottle |
|
Pack Size: |
300ml |
|
Country: |
UK |
|
Date Created: |
24/03/10 |
|
Date Modified: |
04/06/10 |
|
RBH Contact: |
Lori Glasgow |
Artwork Type: Submission Only
Component Code (2D if applicable): RB2894
Bulk Code (if applicable): xxxx
Na
CAD Cam Ref: G-lbl-D0000912-178.5x70mm Printer: Skanem Newcastle
Substrate: Self Adhesive White Paper
Edgemark Position: Na
Pharmacode No/NE: N/A

Print and Technical Colours
PANTONE 320C
PANTONE 2915 C
PANTONE 185C
I PANTONE Reflex Blue C
flx

VRN-HighGlossUV+Opt Briqhtener
PANTONE 116 C
COLOURS TO MATCH RECOGNISED STANDARDS
BARCODE INFO
Barcode Type: Not Known
Barcode Number: N/A
Magnification: 85°/o
BWR: 15 microns
Truncated by (smallest Bar): -19 5 mm Encoded Data:_N/A_
All other aspects of the bar code to conform to the current GS1 Operating Manual or other agreed commercial standard.

TRIDENT
Connaught House, Connaught Road, Kingswood Business Park, Hull, HU7 3AP, England. T: +44 (0) 1482 828100
na - English Alphabet
|
• • |
• |
• |
• |
• |
• • |
• |
• |
• |
|
• • |
• |
• |
• |
• |
• | |||
|
• • |
• |
• |
• | |||||
|
• | ||||||||
|
• |
• |
• |
• |
• |
• |
• | ||
|
• |
• |
• |
• |
• |
• | |||
|
• |
• |
• • |
• |
• |
• • | |||
|
• |
• |
• • |
• |
• | ||||
|
• |
• |
• • |
Please note that any low resolution paper Canon colour copies associated with this job should be referred to for content, layout and colour separation only.
STUDIO USE ONLY Jane Haston vi.o
Sm«Art check results: G=0; 0=0; R=0; - MW - 01/04/10 08:38:05
ARTWORK APPROVAL
Approved by Sonoco Trident on behalf of Reckitt Benckiser
Signed:______________________
Date:_______________________
UNDER NO CIRCUMSTANCES SHOULD THIS ARTWORK BE ALTERED WITHOUT PRIOR PERMISSION FROM TRIDENT.
Tel. Alan Smith: +44 (0)1482 710304
' Gaviscon Cool MintLiquTdTmngs reliefTrom tFe pain and" discomfort of heartburn and acid indigestion, which for example c® occtr after mpals o#du#r*pi#gnan#/#
The po4uct belongs to agroupnf medicines celled 'eflux suppressants', whfcleform a pietective laye#on top of the stomach contents to prevent acid escaping from the stomffcIPwlftre it Porks Pi to thtfiod pi|fe v#i#e it hPts.
RBH Artwork
|
and Print Specification | |
|
Trident Reference No: TR361367 | |
|
Action / Version: |
c |
|
Brand: |
Gaviscon |
|
Brand Platform: |
Adult |
|
Sub Platform: |
Cool |
|
Sub Brand: |
Cool Liquid |
|
Format: |
Liquid |
|
Delivery: |
Bottle |
|
Pack Size: |
300ml |
|
Country: |
UK |
|
Date Created: |
24/03/10 |
|
Date Modified: |
04/06/10 |
|
RBH Contact: |
Lori Glasgow |
Artwork Type: Submission Only
Component Code (2D if applicable): RB2894
EcPh 10ml dos^bf oral sfspfhPon inatftOOmf, sodium hyPogen* nckalcium carbonate 160mg as the '“^itcRris methfl (#lj and propyl izoat&s \OTiich mafcause allergic delayl* This product does not
contains sdffium carbonate 267mg actiyjingri (E21(ffpara . reactions (possibl. contain sugar or gluten. You can take this product if you are pregnant or'
3GbAdults’ includ~ingthe^B and children 12 years and over: 10-20ml (two to four 5ml spoonfuls) after meals and at bedtime (up to four times a day). Children under 12 years: Should only be taken on medical advice. If symptoms persist after 7 days consult your doctor. Contains sodium and calcium.
If you have been advised to follow a diet restricted in either of these salts, please consult your doctor before taking this product.__________
e 267mg and c irediftit^Cjil ra^drojffljfhz
Bulk Code (if applicable): xxxx
Na
CAD Cam Ref: G-lbl-D0000912-178.5x70mm Printer: Skanem Newcastle
Substrate: Self Adhesive White Paper
Edgemark Position: Na
Pharmacode No/NE: N/A

Print and Technical Colours
PANTONE 320C
PANTONE 2915 C
PANTONE 185C
I PANTONE Reflex Blue C
flx

VRN-HighGlossUV+Opt Briqhtener
PANTONE 116 C
COLOURS TO MATCH RECOGNISED STANDARDS
BARCODE INFO
Barcode Type: Not Known
Barcode Number: N/A
Magnification: 85°/o
BWR: 15 microns
Truncated by (smallest Bar): -19 5 mm Encoded Data:_N/A_
All other aspects of the bar code to conform to the current GS1 Operating Manual or other agreed commercial standard.

TRIDENT
Connaught House, Connaught Road, Kingswood Business Park, Hull, HU7 3AP, England. T: +44 (0) 1482 828100
na - English Alphabet
|
• • |
• |
• |
• |
• |
• • |
• |
• |
• |
|
• • |
• |
• |
• |
• |
• | |||
|
• • |
• |
• |
• | |||||
|
• | ||||||||
|
• |
• |
• |
• |
• |
• |
• | ||
|
• |
• |
• |
• |
• |
• | |||
|
• |
• |
• • |
• |
• |
• • | |||
|
• |
• |
• • |
• |
• | ||||
|
• |
• |
• • |
Please note that any low resolution paper Canon colour copies associated with this job should be referred to for content, layout and colour separation only.
STUDIO USE ONLY Jane Haston vi.o
Sm«Art check results: G=0; 0=0; R=0; - MW - 01/04/10 08:38:05
ARTWORK APPROVAL
Approved by Sonoco Trident on behalf of Reckitt Benckiser
Signed:______________________
Date:_______________________
UNDER NO CIRCUMSTANCES SHOULD THIS ARTWORK BE ALTERED WITHOUT PRIOR PERMISSION FROM TRIDENT.
Tel. Alan Smith: +44 (0)1482 710304
|
• • |
• |
• |
• |
• |
• • |
• |
• • | ||
|
• • |
• |
• |
• |
• |
• | ||||
|
• • |
• |
• |
• | ||||||
|
• | |||||||||
|
• |
• |
• |
• |
• |
• • | ||||
|
• |
• |
• |
• |
• |
• | ||||
|
• |
• |
• • |
• |
• |
• • | ||||
|
• |
• |
• • |
• |
• | |||||
|
• |
• |
• • |
|
Trident Reference No: |
TR361367 |
|
Action / Version: |
C |
|
Brand: |
Gaviscon |
|
Brand Platform: |
Adult |
|
Sub Platform: |
Cool |
|
Sub Brand: |
Cool Liquid |
|
Format: |
Liquid |
|
Delivery: |
Bottle |
|
Pack Size: |
300ml |
|
Country: |
UK |
|
Date Created: |
24/03/10 |
|
Date Modified: |
04/06/10 |
rbh Contact: |_ori Glasgow
Artwork Type: Submission Only
Component Code (20 if applicable): RB2894
Parent Technical Packaging Specification:
Bulk Code (If applicable): N3
xxxx
CAD Cam Ref: G~lbl-D0000912~178.5x70mm Printer: Skanem Newcastle
Substrate: Self Adhesive White Paper
Edgemark Position: Na
Pharmacode No/NE: N/A
|
Cutter - Does rot print |
Guides- Does not print |
|
I |
PRINTING | |
|
READS | ||
|
NJ to u7 |
This Way |
GT
Print and Technical Colours
|
PANTONE 320 C |
PANTONE Reflex Blue C |
VRN-HighGlossUV+Opt Briqhtener | |||
|
1 FLX |
2 FLX |
3 FLX | |||
|
PANTONE 2915 C |
Braille |
PANTONE 116 C | |||
|
4 FIX |
5 |
6 FLX | |||
|
PANTONE 185 C | |||||
|
7 FLX | |||||
COLOURS TO MATCH RECOGNISED STANDARDS
|
Barcode Type: |
Not Known |
|
Barcode Number: |
N/A |
|
Magnification: |
85% |
|
BWR: |
15 microns |
|
Truncated by (smallest Bar): |
-19.5 mm |
|
Encoded Data: |
N/A |
All other aspects of the bar code to conform to the current GSl Operating Manual or other agreed commercial standard.
TRIDENT
Connaught Bouse, Connaught Road, Kingswood Business Park, Bull, BU7 3AP, England. T: +44 (0) 1482 828100
|
na - English Alphabet | ||||||
|
[J| | ||||||
|
Urn | ||||||
|
II |
B | |||||
Please note that any low resolution paper Canon colour copies associated with this job should be referred to for content, layout and colour separation only.
STUDIO USE ONLY Jane Haston vi.o
Sm»Art check results: G=0;0=0; R=0; - MW -01/04/10 08:38X55
Approved by Sonoco Trident on behalf of Reckitt Benckiser
Signed:______________________
Date:_______________________
UNDER NO CIRCUMSTANCES SBOULD THIS ARTWORK BE ALTERED W1TBOUT PRIOR PERMISSION FROM TRIDENT.
Tel. Alan Smith: +44 (0)1482 710304
RBH Artwork and Print Specification
|
Trident Reference No: |
TR361367 |
|
Action / Version: |
C |
|
Brand: |
Gaviscon |
|
Brand Platform: |
Adult |
|
Sub Platform: |
Cool |
|
Sub Brand: |
Cool Liquid |
|
Format: |
Liquid |
|
Delivery: |
Bottle |
|
Pack Size: |
300ml |
|
Country: |
UK |
|
Date Created: |
24/03/10 |
|
Date Modified: |
04/06/10 |
rbh Contact: |_ori Glasgow
Artwork Type: Submission Only
Component Code (20 if applicable): RB2894
Parent Technical Packaging Specification:
Bulk Code (If applicable): N3
xxxx
CAD Cam Ref: G~lbl-D0000912~178.5x70mm Printer: Skanem Newcastle
Substrate: Self Adhesive White Paper
Edgemark Position: Na
Pharmacode No/NE: N/A
|
1 |
PRINTING | |
|
READS | ||
|
NJ to u7 |
This Way |
GT
Print and Technical Colours
|
PANTONE 320 C |
PANTONE Reflex Blue C |
VRN-HighGlossUV+Opt Briqhtener | |||
|
1 FLX |
2 FLX |
3 FLX | |||
|
PANTONE 2915 C |
Braille |
PANTONE 116 C | |||
|
4 FIX |
5 |
6 FLX | |||
|
PANTONE 185 C | |||||
|
7 FLX | |||||
|
Cutter - Does rot print |
Guides- Does not print |
COLOURS TO MATCH RECOGNISED STANDARDS
BARCODE INTO
All other aspects of the bar code to conform to the current GSl Operating Manual or other agreed commercial standard.
TRIDENT
Connaught Bouse, Connaught Road, Kingswood Business Park, Bull, BU7 3AP, England. T: +44 (0) 1482 828100
|
na - English Alphabet | ||||||
|
[J| | ||||||
|
Urn | ||||||
|
II |
B | |||||
Please note that any low resolution paper Canon colour copies associated with this job should be referred to for content, layout and colour separation only.
STUDIO USE ONLY Jane Haston vi.o
Sm»Art check results: G=0;0=0; R=0; - MW -01/04/10 08:38X55
|
Barcode Type: |
Not Known |
|
Barcode Number: |
N/A |
|
Magnification: |
85% |
|
BWR: |
15 microns |
|
Truncated by (smallest Bar): |
-19.5 mm |
|
Encoded Data: |
N/A |
Approved by Sonoco Trident on behalf of Reckitt Benckiser
Signed:______________________
Date:_______________________
UNDER NO CIRCUMSTANCES SBOULD THIS ARTWORK BE ALTERED W1TBOUT PRIOR PERMISSION FROM TRIDENT.
Tel. Alan Smith: +44 (0)1482 710304
uojtsdBjpui 9 ujnqyedH • jnoABjj tujifl /ooj

Do not store above 30°C. Do not refrigerate or freeze. Keep out of the reach and sight of children.
■Manufacturing Authorisation Holder in UK:
[Reckitt Benckiser Healthcare (UK) Ltd., Hull, HU8 7DS. [Gaviscon and the sword 8i circle are trade marks.
0 0
[Gaviscon Cool Mint Liquid brings relief from the pain ■and discomfort of heartburn and acid indigestion,
Which for example, can occur after meals or during [pregnancy. The product belongs to a group of medicines called 'reflux suppressants', which form a protective layer on top of the stomach contents to [prevent acid escaping from the stomach where it works into the food pipe where it hurts.
[ Each 10ml dose of oral
[suspension contains sodium alginate 500mg, sodium [hydrogen carbonate 267mg and calcium carbonate <160mg as the active ingredients. Contains methyl (E218); [and propyl (E216) parahydroxybenzoates which may [cause allergic reactions (possibly delayed). This ■product does not contain sugar or gluten. You can take [this product if you are pregnant or breast feeding.
[ Read the
■package leaflet before use. Adults, including the [elderly and children 12 years and over: 10-20ml (two to [four 5ml spoonfuls) after meals and at bedtime (up to [four times a day). Children under 12 years: Should only [be taken on medical advice. If symptoms persist after 7 [days consult your doctor. Contains sodium and [calcium. If you have been advised to follow a diet restricted in either of these salts, please consult your [doctor before taking this product.
[Medicines should not be disposed of via wastewater oi[ household waste. Ask your pharmacist how to dispose [of medicines no longer required. These measures will [help to protect the environment.
■PL00063/0158
>
TBRXXX - English Alphabet
TECHNICAL INFO:
Repro not applied and PPM check required at commercial print stage
RBH Artwork and Print Specification
|
Trident Reference No: |
TR361271 |
|
Action / Version: |
C |
|
Brand: |
Gaviscon |
|
Brand Platform: |
Adult |
|
Sub Platform: |
Cool |
|
Sub Brand: |
Cool Liquid |
|
Format: |
Liquid |
|
Delivery: |
Bottle |
|
Pack Size: |
300ml |
|
Country: |
UK |
|
Date Created: |
29/03/10 |
|
Date Modified: |
01/06/10 |
Lori Glasgow
Artwork Type: Submission Only
Component Code (2D if applicable): RB2895
“!53329
Bulk Code (if applicable): NA
xxxx
CAD Cam Ref: G-Crt-D0253329-70x70x160mm Printer: Nampak Healthcare (Previously Storey Evans)
Substrate: Carton Board - White
Edgemark Position: NA
Pharmacode No/NE: N/A

Print and Technical Colours
PANTONE 320C
PANTONE 102C
I PANTONE Reflex Blue C LTH
PANTONE 185 C

VRN-HighGloss Water Based _Emul
PANTONE 2915 C
COLOURS TO MATCH RECOGNISED STANDARDS
BARCODE INFO
Barcode Type: Not Known
Barcode Number: N/A
Magnification: 100%
BWR: 10 microns
Truncated by (smallest Bar): 0 mm Encoded Data:_N/A_
All other aspects of the bar code to conform to the current GS1 Operating Manual or other agreed commercial standard.

TRIDENT
Connaught House, Connaught Road, Kingswood Business Park, Hull, HU7 3AP, England. T; +44 (0) 1482 828100
Please note that any low resolution paper Canon colour copies associated with this job should be referred to for content, layout and colour separation only.
STUDIO USE ONLY Jane Haston vi.o
Sm«Art check results: G=0; 0=0; R=0; - JH - 01/06/10 15:58:49
ARTWORK APPROVAL
Approved by Sonoco Trident on behalf of Reckitt Benckiser
Signed:______________________
Date:_______________________
LINDER NO CIRCUMSTANCES SHOULD THIS ARTWORK BE ALTERED WITHOUT PRIOR PERMISSION FROM TRIDENT.
Tel. Alan Smith: +44 (0)1482 710304
NOT APPROVED FOR COLOUR
uojtsdBjpui 9 ujnqyedH • jnoABjj tujifl /ooj
dieuoqjg) uinpiej 1 d}euoqjeD udBojp/q uinipos ajei//6/e uinipos \NOISN3dSflS IVdO
pm bn
Do not store above 30°C. Do not refrigerate or freeze. Keep out of the reach and sight of children.
■Manufacturing Authorisation Holder in UK:
(Reckitt Benckiser Healthcare (UK) Ltd., Hull, HU8 7DS. (Gaviscon and the sword & circle are trade marks.
0 0
paviscon Cool Mint Liquid brings relief from the pain ■and discomfort of heartburn and acid indigestion,
Which for example, can occur after meals or during pregnancy. The product belongs to a group of medicines called 'reflux suppressants', which form a protective layer on top of the stomach contents to prevent acid escaping from the stomach where it works into the food pipe where it hurts.
! Each 10ml dose of oral
(suspension contains sodium alginate 500mg, sodium hydrogen carbonate 267mg and calcium carbonate (l60mg as the active ingredients. Contains methyl (E218); land propyl (E216) parahydroxybenzoates which may pause allergic reactions (possibly delayed). This ■product does not contain sugar or gluten. You can take this product if you are pregnant or breast feeding.
| Read the
■package leaflet before use. Adults, including the (elderly and children 12 years and over: 10-20ml (two to (four 5ml spoonfuls) after meals and at bedtime (up to four times a day). Children under 12 years: Should only (be taken on medical advice. If symptoms persist after 7 (days consult your doctor. Contains sodium and palcium. If you have been advised to follow a diet restricted in either of these salts, please consult your (doctor before taking this product.
(Medicines should not be disposed of via wastewater oi( household waste. Ask your pharmacist how to dispose (of medicines no longer required. These measures will help to protect the environment.
■PL00063/0158
>
TBRXXX - English Alphabet
TECHNICAL INFO:
Repro not applied and PPM check required at commercial print stage
RBH Artwork and Print Specification
|
Trident Reference No: |
TR361271 |
|
Action / Version: |
C |
|
Brand: |
Gaviscon |
|
Brand Platform: |
Adult |
|
Sub Platform: |
Cool |
|
Sub Brand: |
Cool Liquid |
|
Format: |
Liquid |
|
Delivery: |
Bottle |
|
Pack Size: |
300ml |
|
Country: |
UK |
|
Date Created: |
29/03/10 |
|
Date Modified: |
01/06/10 |
Lori Glasgow
Artwork Type: Submission Only
Component Code (2D if applicable): RB2895
“!53329
Bulk Code (if applicable): NA
xxxx
CAD Cam Ref: G-Crt-D0253329-70x70x160mm Printer: Nampak Healthcare (Previously Storey Evans)
Substrate: Carton Board - White
Edgemark Position: NA
Pharmacode No/NE: N/A

Print and Technical Colours
PANTONE 320C
PANTONE 102C
I PANTONE Reflex Blue C LTH
PANTONE 185 C

VRN-HighGloss Water Based _Emul
PANTONE 2915 C
COLOURS TO MATCH RECOGNISED STANDARDS
BARCODE INFO
Barcode Type: Not Known
Barcode Number: N/A
Magnification: 100%
BWR: 10 microns
Truncated by (smallest Bar): 0 mm Encoded Data:_N/A_
All other aspects of the bar code to conform to the current GS1 Operating Manual or other agreed commercial standard.

TRIDENT
Connaught House, Connaught Road, Kingswood Business Park, Hull, HU7 3AP, England. T; +44 (0) 1482 828100
Please note that any low resolution paper Canon colour copies associated with this job should be referred to for content, layout and colour separation only.
STUDIO USE ONLY Jane Haston vi.o
Sm«Art check results: G=0; 0=0; R=0; - JH - 01/06/10 15:58:49
ARTWORK APPROVAL
Approved by Sonoco Trident on behalf of Reckitt Benckiser
Signed:______________________
Date:_______________________
LINDER NO CIRCUMSTANCES SHOULD THIS ARTWORK BE ALTERED WITHOUT PRIOR PERMISSION FROM TRIDENT.
Tel. Alan Smith: +44 (0)1482 710304
NOT APPROVED FOR COLOUR
TECHNICAL INFO:
Repro not applied and PPM check required at commercial print stage
RBH Artwork and Print Specification
|
Trident Reference No: |
TR361271 |
|
Action / Version: |
C |
|
Brand: |
Gaviscon |
|
Brand Platform: |
Adult |
|
Sub Platform: |
Cool |
|
Sub Brand: |
Cool Liquid |
|
Format: |
Liquid |
|
Delivery: |
Bottle |
|
Pack Size: |
300ml |
|
Country: |
UK |
|
Date Created: |
29/03/10 |
|
Date Modified: |
01/06/10 |
Lori Glasgow
Artwork Type: Submission Only
Component Code (2D if applicable): RB2895
D0253329
NA
Bulk Code (if applicable]:
xxxx
CAD Cam Ref: G-Crt-D0253329-70x70x160mm Printer: Nampak Healthcare (Previously Storey Evans)
Substrate: Carton Board - White
Edgemark Position: NA
Pharmacode No/NE: N/A
|
Cutter - Does rot print |
Guides- Does not print |
Print and Technical Colours
|
PANTONE 320 C |
PANTONE Reflex Blue C |
VRN-HighGloss Water Based Emiil | |||
|
1 |
2 |
3 | |||
|
LTH |
LTH |
LTH | |||
|
PANTONE 102 C |
PANTONE 185 C |
PANTONE 2315 C | |||
|
4 |
5 |
6 | |||
|
LTH |
LTH |
LTH | |||
COLOURS TO MATCH RECOGNISED STANDARDS
BARCODE 11NEO
Barcode Type: Not Known
Barcode Number: N/A
Magnification: 100%
BWR: 10 microns
Truncated by (smallest Bar): Omm Encoded Data:_N/A_
All other aspects of the bar code to conform to the current GS1 Operating Manual or other agreed commercial standard.
TRIDENT
Connaught House, Connaught Road, Kingswood Business Park, Hull, HTJ7 3AP, Engl and. T: +44 (0) 1482 828100
Please note that any low resolution paper Canon colour copies associated with this job should be referred to for content, layout and colour separation only.
STUDIO USE ONLY Jane Haston v1.0
Sm«Art check results: G=0; 0=0; R=0; - JH - 01/06/1015:58:49
ARTWORK APPROVAL
Approved by Sonoco Trident on behalf of Reckitt Benckiser
Signed:______________________
Date:_______________________
UNDER NO CIRCUMSTANCES SHOULD THIS ARTWORK BE ALTERED WITHOUT PRIOR PERMISSION FROM TRIDENT.
Tel. Alan Smith: +44 (0)1482 710304
NOT APPROVED FOR COLOUR
TBRXXX - English Alphabet
|
• • • |
• • |
• • |
|
• • |
• |
• • |
|
• • •• |
• • |
• • |
|
• • •• |
• |
• |
|
• • |
• • |
• • • •• • •• • • • • • • •• • • • • • •
TECHNICAL INFO:
Repro not applied and PPM check required at commercial print stage
RBH Artwork and Print Specification
|
Trident Reference No: |
TR361271 |
|
Action / Version: |
C |
|
Brand: |
Gaviscon |
|
Brand Platform: |
Adult |
|
Sub Platform: |
Cool |
|
Sub Brand: |
Cool Liquid |
|
Format: |
Liquid |
|
Delivery: |
Bottle |
|
Pack Size: |
300ml |
|
Country: |
UK |
|
Date Created: |
29/03/10 |
|
Date Modified: |
01/06/10 |
Lori Glasgow
Artwork Type: Submission Only
Component Code (2D if applicable): RB2895
ST1* D0253329
Bulk Code (if applicable]: NA
xxxx
CAD Cam Ref: G-Crt-D0253329-70x70x160mm Printer: Nampak Healthcare (Previously Storey Evans)
Substrate: Carton Board - White
Edgemark Position: NA
Pharmacode No/NE: N/A
|
Cutter - Does rot print |
Guides- Does not print |
Print and Technical Colours
|
PANTONE 320 C |
PANTONE Reflex Blue C |
VRN-HighGloss Water Based Efflul | |||
|
1 |
2 |
3 | |||
|
LTH |
LTH |
LTH | |||
|
PANTONE 102 C |
PANTONE 185 C |
PANTONE 2315 C | |||
|
4 |
5 |
6 | |||
|
LTH |
LTH |
LTH | |||
COLOURS TO MATCH RECOGNISED STANDARDS
BARCODE 11NIE0
Barcode Type: Not Known
Barcode Number: N/A
Magnification: 100%
BWR: 10 microns
Truncated by (smallest Bar): 0 mm Encoded Data:_N/A_
All other aspects of the bar code to conform to the current GS1 Operating Manual or other agreed commercial standard.

TRIDENT
Connaught House, Connaught Road, Kingswood Business Park, Hull, HTJ7 3AP, Engl and. T: +44 (0) 1482 828100
Please note that any low resolution paper Canon colour copies associated with this job should be referred to for content, layout and colour separation only.
STUDIO USE ONLY Jane Haston v1.0
Sm«Art check results: G=0; 0=0; R=0; - JH - 01/06/1015:58:49
ARTWORK APPROVAL
Approved by Sonoco Trident on behalf of Reckitt Benckiser
Signed:______________________
Date:_______________________
UNDER NO CIRCUMSTANCES SHOULD THIS ARTWORK BE ALTERED WITHOUT PRIOR PERMISSION FROM TRIDENT.
Tel. Alan Smith: +44 (0)1482 710304
NOT APPROVED FOR COLOUR
TBRXXX - English Alphabet
|
• • • |
• • |
• • |
|
• • |
• |
• • |
|
• • •• |
• • |
• • |
|
• • •• |
• |
• |
|
• • |
• • |
• • • •• • •• • • • • • • •• • • • • • •
Gaviscon Cool MintXTquTd.
podium alginate, sodium hydrogen carbonate and calcium carbonate.
'Read all of this leaflet carefully because it contains important information for you.
[This medicine is available without prescription. However,you still need to take this medicine carefully to get the best results from it. 1
b Keep this leaflet. You may need to read it againj.
|* Ask your pharmacist if you need more information or advice, r You must contact a doctor if your symptoms Worsen or do not improve after 7 days.
>• If any of the side-effects gets serious, or if you ijiotice any side-effect not listed in this leaflet,
| please tell your doctor or pharmacist.
In this leaflet:
|1. What is Gaviscon Cool Mint Liquid and what is lit used for?
2. Before you take Gaviscon Cool Mint Liquid.
8. How to take Gaviscon Cool Mint Liquid.
|4. Possible side effects.
,5. How to store Gaviscon Cool Mint Liquid.
6. Further information.
|l. WHAT IS GAVISCON COOL MINT LIQUID AND WHAT IS IT USED FOR?
Gaviscon Cool Mint Liquid belongs to a group of medicines called "reflux suppressants" which form a protective layer on top of the stomach contents to prevent stomach acid escaping from the stomach where it works into the food pipe causing pain and discomfort.
'This medicine is used for the treatment of symptoms of gastro-oesophageal reflux such as acid 'regurgitation, heartburn and indigestion (related |to reflux),for example,following meals, or during pregnancy, or in patients with symptoms related to reflux oesophagitis.
|2. BEFORE YOU TAKE GAVISCON COOL MINT LIQUID iDo not take Gaviscon Cool Mint Liquid '
L if you know that you are allergic (hypersensitive] to any of the ingredients of Gaviscon Cool Mint [Liquid as very rarely difficulty in breathing and skin rashes have occurred (see further information ifor a full list). '
[Take special care with Cool Mint Liquid i
This medicine contains small amounts of sodium (6.2 mmol per 10 ml) and calcium '(1.6 mmol per 10 ml).
]- If you have been advised to follow a diet restricted in any of these please consult your doctor, r Please also talk to your doctor regarding these salt contents if you suffer or have suffered from significant kidney or heart disease, as certain salt^ could interfere with these diseases, please consult your doctor if you know you have reduced amounts of gastric acid in your stomach, as this product may be less effective.
'If symptoms persist after 7 days consult your doctor.
'Taking other medicines:
'Do not take this product within two hours of taking other medicines by mouth as it can interfere iwith the action of some other medicines. '
'Please tell your doctor or pharmacist if you are tajeing or have recently taken any other medicines jincluding medicines obtained without prescription.
'Pregnancy and breast-feeding:
you can take this product if you are pregnant or breast-feeding.
RB2896
FiE©
Important information about some of the Ingredients of Gaviscon Gobi Mint Liquid
[This product contains methyl (E218) and propyl (£216) para-hydroxybenzoates which may cause
allergic reactions (possibly delayed). i
[3. HOW TO TAKE GAVISCON COOL MINT (.IQUID
[Shake well before use.
Adults, including the elderly and children l[2 years and over: 10-20 ml after meals and at
bedtime (up to four times a day). i
[Children under 12 years: Should only be taker] on medical advice.
If you take more Gaviscon Cool Mint Liquid'than you should
[if you take too much of this product it is unlikely |o cause you any harm. However, you may feel bloated. Consult your doctor If this doesn't go away.
[If you forget to take Gaviscon Cool Mint Liduid
If you forget a dose it is not necessary to double tpe dose next time, just carry on taking as before. [4. POSSIBLE SIDE EFFECTS [
Like all medicines, Gaviscon Cool Mint Liquid can cause side effects, although not everybody gets them. 1
If you experience these side effects stop taking the product and consult your doctor immediately. Very rare (less than 1 in 10,000) chance of an allergic reaction to the ingredients. Symptoms of this [may include skin rash, itching, difficulty breathing, dizziness, or swelling of the face, lips, tongue or throat. i
}f any of the side effects gets serious, or you notiefe any side effects not listed in this leaflet, please itell your doctor or pharmacist.
[5. HOWTO STORE GAVISCON COOL MINT LIQUID KEEP OUT OF THE REACH AND SIGHT OF CHILDREN
Do not use Gaviscon Cool Mint Liquid after the expiry date which is stated on the label {EXP: [month/yearl.The expiry date refers to the last dajf of that month.
Do not store above 30°C. Do not freeze or refrigerate.
Check that the cap seal is unbroken before first using this product.
[Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist bow to dispose of medicines no longer required.These measures will help to protect the bnvironment. '
6. FURTHER INFORMATION
What Gaviscon Cool Mint Liquid contains 1
[The active substances in each 10 ml of oral suspension contains 500 mg sodium alginate, 267 mg
sodium hydrogen carbonate and 160 mg calcium carbonate as the active ingredients.The other
Ingredients are carbomer, methyl (E218) and propyl (E216) parahydroxy-benzoates, saccharin
podium, sodium hydroxide, mint flavour no.4, mirjt flavour no. 5 and purified water.This product
does not contain sugar or gluten. i
[What Gaviscon Cool Mint Liquid looks like ^nd contents of the pack
Gaviscon Cool Mint Liquid is an off-white suspension with the odour and flavour of peppermint.
Gaviscon Cool Mint Liquid is available in bottles df 150 ml or 300 ml.
Marketing Authorisation Holder and Manufacturer
Reckitt Benckiser Healthcare (UK) Limited, Dansoih Lane, Hull, HU8 7DS.
I |
This medicinal product is authorised in the member states of the EA under the following names:
[UK: Gaviscon Cool Mint Liquid.
[This leaflet was last approved in February 2010.
RB2896 1
FREE
-AREA
CUSTOMER INFO: Minimum Point Size = 7 pt
RBH Artwork
|
and Print Specification | |
|
Trident Reference No: TR361350 | |
|
Action / Version: |
B |
|
Brand: |
Gaviscon |
|
Brand Platform: |
Adult |
|
Sub Platform: |
Cool |
|
Sub Brand: |
Cool Liquid |
|
Format: |
Liquid |
|
Delivery: |
Bottle |
|
Pack Size: |
300ml |
|
Country: |
UK |
|
Date Created: |
24/03/10 |
|
Date Modified: |
31/03/10 |
|
RBH Contact: |
Lori Glasgow |
Artwork Type: Submission Only
Component Code (2D if applicable): RB2896
Parent Technical Packaging Specification:
Bulk Code (if applicable]: NS
xxxx
CAD Cam Ref: G-Lflt-D0125806-148x210mm Printer: Nampack Healthcare (Previously Storey Evans)
Substrate: Paper - White
Edgemark Position: Na Pharmacode No/NE: N/A
|
Cutter - Does not print |
Guides-Does not print |
Print and Technical Colours

COLOURS TO MATCH RECOGNISED STANDARDS
BARCODE INFO
|
Barcode Type: |
Not Known |
|
Barcode Number: |
N/A |
|
Magnification: |
N/A |
|
BWR: |
N/A |
|
Truncated by (smallest Bar): |
N/A |
|
Encoded Data: |
N/A |
All other aspects of the bar code to conform to the current GS1 Operating Manual or otheT agreed commercial standard.

TRIDENT
Connaught house, Connaught Road, Kingswood Business Paik, hull, HU7 3AP, England. T: +44 (0) 1482 828100
Please note that any low resolution paper Canon colour copies associated with this job should be referred to for content, layout and colour separation only.
STUDIO USE ONLY Monika Whitehead vi.o
Sm*Art check results: G=0; 0=0; R=0; - MW - 31/03/10 11:25:05
ARTWORK APPROVAL
Approved by Sonoco Trident on behalf of Reckitt Benckiser
Signed:______________________
Date:_______________________
UNDER NO CIRCUMSTANCES SHOULD THIS ARTWORK BE ALTERED WITHOUT PRIOR PERMISSION FROM TRIDENT.
Tel. Alan Smith: +44 (0)1482 710304
'GavisconToollMmtTiquiTbnngs refieffromlhe pain and 'discomfort of heartburn and acid indigestion, which for j example, can occur after meals or during pregnancy. The product belongs to a group of medicines called 'reflux isuppressants', which form a protective layer on top of the istomach contents to prevent acid escaping from the istomach where it works into the food pipe where it hurts, i JJjjijmiTg W Each 10ml dose of oral suspension ■ contains sodium alginate 500mg, sodium hydrogen 'carbonate 267mg and calcium carbonate 160mg as the 'active ingredients. Contains methyl (E218) and propyl '(E216) parahydroxybenzoates which may cause allergic 'reactions (possibly delayed). This product does not .contain sugar or gluten. You can take this product if you ,are pregnant or breast feet"
Do not store above 30°C. Do not refrigerate or freeze.
Keep out of the reach and sight of children
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
Manufacturer and Product Licence Holder in UK:
Reckitt Benckiser Healthcare (UK) Ltd., Hull, HU8 7DS. Gaviscon and the sword & circle are trade marks. RB002888 PL00063/0158'
i Adults, including the elderly
■ and children 12 years and over: 10-20ml (two to four 5ml 'spoonfuls) after meals and at bedtime, (up to four times 'a day). Children under 12 years: Should only be taken on 'medical advice. If symptoms persist after 7 days consult 'your doctor. Contains sodium and calcium. If you have been | advised to follow a diet restricted in either of these salts, .please consult your doctor before taking this product.
_P eeLhere^Dp notremove. 5QjTI.

Pharmacode Reads This Way

Last Bar
First Bar
RBH Artwork
|
and Print Specification | |
|
Trident Reference No: TR360542 | |
|
Action / Version: |
C |
|
Brand: |
Gaviscon |
|
Brand Platform: |
Adult |
|
Sub Platform: |
Cool |
|
Sub Brand: |
Cool Liquid |
|
Format: |
Liquid |
|
Delivery: |
Bottle |
|
Pack Size: |
150ml |
|
Country: |
UK |
|
Date Created: |
23/03/10 |
|
Date Modified: |
01/04/10 |
|
RBH Contact: |
Lori Glasgow |
Artwork Type: Submission Only
Component Code (2D if applicable): RB002888
P“cka3lna
Bulk Code (if applicable): N/a
XXXX
CAD Cam Ref: G-lbl-D0006045-155x60mm - P&R Printer: Regulatory Artwork - No Printer Required
Substrate: Self Adhesive White Paper
Edgemark Position: N/a
Pharmacode No/NE: n/a

Print and Technical Colours
PANTONE Reflex Blue C
Gaviscon Coo nt quid.
Sodium alginate, sodium hydrogen carbonate and 'calcium carbonate.
Read all of this leaflet carefully because it contains important information for you.
This medicine is available without prescription. However, you still need to take this medicine carefully jto get the best results from it. p Keep this leaflet. You may need to read it again.
Askvour pharmacist if you need more information 1 or advice.
) You must contact a doctor if your symptoms i worsen or do not improve after 7 days.
If any of the side-effects gets serious, or if you 1 notice any side-effect not listed in this leaflet,
| please tell your doctor or pharmacist.
,ln this leaflet:
1. What is Gaviscon Cool Mint Liquid and what is it used for?
2. Before you take Gaviscon Cool Mint Liquid.
R. How to take Gaviscon Cool Mint Liquid.
i Possible side effects.
5. Howto store Gaviscon_Cool_Mint_Liq_uid.____
"6. Further information. "
1. WHAT IS GAVISCON COOL MINT LIQUID AND WHATISITUSEDFOR?
Gaviscon Cool Mint Liquid belongs to a group of medicines called "reflux suppressants", which form a protective layer on top of the stomach contents to prevent stomach acid escaping from the stomach where it works into the food pipe causing pain and discomfort.
This medicine is used for the treatment of symptoms of gastro-oesophageal reflux such as acid regurgitation, heartburn and indigestion (related to reflux), for example, following meals, or during pregnancy, or in patients with symptoms related to reflux oesophagitis.
2. BEFORE YOU TAKE GAVISCON COOL MINT LIQUID Do not take Gaviscon Cool Mint Liquid
- if you know that you are allergic (hypersensitive) to any of the ingredients of Gaviscon Cool Mint Liquid as very rarely difficulty in breathing and skin rashes have occurred (see further information for a full list). Take special care with Cool Mint Liquid This medicine contains small amounts of sodium (6.2
mmol"per IT ml) and"calcium (1.6 mmofper 10 ml).
- If you have been advised to follow a diet restricted in any of these please consult your doctor.
- Please also talk to your doctor regarding these salt contents if you suffer or have suffered from significant kidney or heart disease, as certain salts could interfere with these diseases.
Please consult your doctor ifyou knowyou have reduced amounts of gastric acid in your stomach, as this product may be less effective.
If symptoms persist after 7 days consult your doctor. Taking other medicines:
Do not take this product within two hours of taking other medicines by mouth as it can interfere with the action of some other medicines.
Please tell your doctor or pharmacist ifyou are taking or have recently taken any other medicines including medicines obtained without prescription. Pregnancy and breast-feeding:
You can take this product ifyou are pregnant or breast-feeding.
Important information about some of the ingredients of Gaviscon C_oo_[MinUiquid____RB002888

Reflex Blue Tone

PANTONE 116 C
Varnish Generic
PANTONE 185 C
PANTONE 320 C
|
4 FLX | |
|
PANTONE 2915 C | |
|
7 FLX | |
Colours to m atch recognised s tandards
BARCODE INFO
|
Barcode Type: |
Not Known |
|
Barcode Number: |
N/A |
|
Magnification: |
N/A |
|
BWR: |
N/A |
|
Truncated by (smallest Bar): |
N/A |
|
Encoded Data: |
N/A |
All other aspects of the bar code to conform to the current GS1 Operating Manual or other agreed commercial standard.
This product contains methylTEZWand propyl(E2T6)' para-hydroxybenzoates which may cause allergic reactions (possibly delayed).
3. HOWTOTAKE GAVISCON COOL MINT LIQUID For oral administration. Shake well before use.
Adults, including the elderly and children 12 years and over: 10-20 ml after meals and at bedtime (up to four times a day).
Children under 12 years: Should only be taken on medical advice.
If you take more Gaviscon Cool Mint Liquid than you should
Ifyou take too much of this product it is unlikely to cause you any harm. However, you may feel bloated. Consultyour doctor if this doesn't go away.
If you forget to take Gaviscon Cool Mint Liquid Ifyou forget a dose it is not necessary to double the dose next time, just carry on taking as before.
4. POSSIBLE SIDE EFFECTS
Like all medicines, Gaviscon Cool Mint Liquid can cause side effects, although not everybody gets them.
If_you experience thesejide effects s_topta_king the_
product an consultyour doctor immediately. Very rare (less than 1 in 10,000) chance of an allergic reaction to the ingredients. Symptoms of this may include skin rash, itching, difficulty breathing, dizziness, or swelling of the face, lips, tongue or throat.
If any of the side effects gets serious, or you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
5. HOW TO STORE GAVISCON COOL MINT LIQUID KEEP OUT OF THE REACH AND SIGHT OF CHILDREN Do not use Gaviscon Cool Mint Liquid afterthe expiry date which is stated on the label {EXP: month/year}. The expiry date refers to the last day of that month.
Do not store above 30°C. Do not freeze or refrigerate Checkthatthe cap seal is unbroken before first using this product.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist howto dispose of medicines no longer required. These measures will help to protect the environment.
6. FURTHER INFORMATION
What_Ga_visco_n Coo| Mint LjqridMntains____
The active stances in eacT TO ml o ora suspension contains 500 mg sodium alginate, 267 mg | sodium hydrogen carbonate and 160 mg calcium i carbonate as the active ingredients. The other ■ ingredients are carbomer, methyl (E218) and propyl 1 (E216) parahydroxy-benzoates, saccharin sodium, j sodium hydroxide, mint flavour no. 4, mint flavour no. i 5 and purified water. This product does not contain i sugar or gluten.
What Gaviscon Cool Mint Liquid looks like and | contents of the pack .
Gaviscon Cool Mint Liquid is an off-white suspension! with the odour and flavour of peppermint. 1
Gaviscon Cool Mint Liquid is available in bottles of ' 150 ml or 300 ml. .
Marketing Authorisation Holder and Manufacturer Reckitt Benckiser Healthcare (UK) Limited, Dansom ■ Lane, Hull, HU87DS. 1
This medicinal product is authorised in the member | states of the EA under the following names: i
UK: Gaviscon Cool Mint Liquid. ■
This leaflet was last approved in February 2010. 1
BB0.02888i
£3
TRIDENT
Connaught House, Connaught Road, Kingswood Business Park, Hull, HU7 3AP, England. T: +44 (0) 1482 828100
Please note that any low resolution paper Canon colour copies associated with this job should be referred to for content, layout and colour separation only.
STUDIO USE ONLY Lee Hardey vi.o
Sm^Art check results: G=0; O=0; R=0; - LH - 01/04/10 11:03:26
ARTWORK APPROVAL
Approved by Sonoco Trident on behalf of Reckitt Benckiser
Signed:______________________
Date:_______________________
CUSTOMER INFO: Minimum Point Size = 6 pt Horizontal scale = 78%
UNDER NO CIRCUMSTANCES SHOULD THIS ARTWORK BE ALTERED WITHOUT PRIOR PERMISSION FROM TRIDENT.
Tel. Alan Smith: +44 (0)1482 710304
NOT APPROVED FOR COLOUR