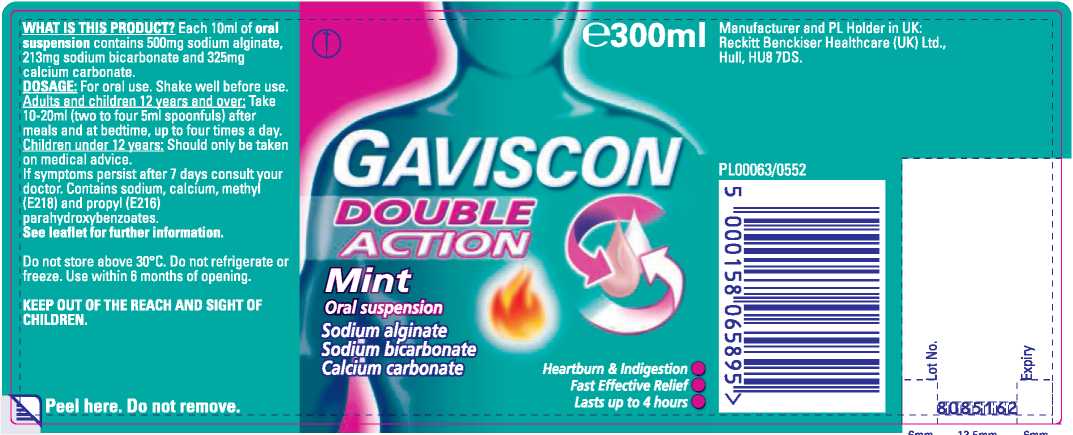
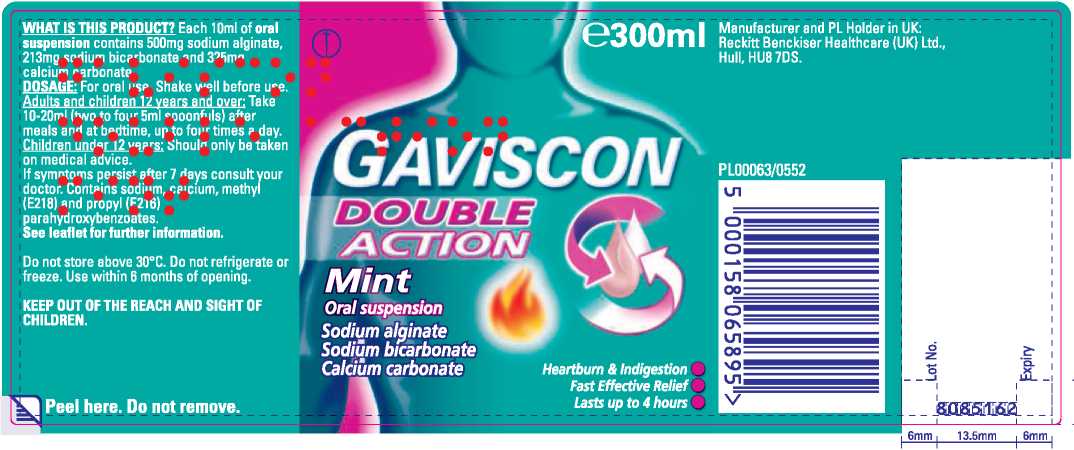
Gaviscon Double Action Mint
155mm
Each 10ml of oral suspension contains 500mg sodium alginate, 213mg sodium bicarbonate and 325mg calcium carbonate. DOSAGE: For oral use. Shake well before use. Adults and children 12 years and over: Take 10-20ml (two to four 5ml spoonfuls) after
■ '■
(GAVISCON
Do not store above 30°C.
Do not refrigerate or freeze. KEEP OUT OF THE REACH AND SIGHT OF CHILDREN.
Use within 6 months of opening.
Manufacturer and PL Holder in UK:
Reckitt Benckiser Healthcare (UK) Ltd., Hull, HU87DS.
150ml


PATIENT INFORMATION LEAFLET GAVISCON DOUBLE ACTION MINT
Sodium alginate, Sodium bicarbonate, Calcium carbonate
Please read this leaflet carefully before you take this medicine. If you are not sure about anything ask your pharmacist or doctor.
What is Gaviscon Double Action Mint and what is it used for?
Gaviscon Double Action Mint is a combination of two antacids (calcium carbonate and sodium bicarbonate) and an alginate and works in two ways:
1) Neutralising excess stomach acid to relieve the pain and discomfort.
2) Forming a protective barrier over the stomach contents to soothe the burning pain in your chest.
Gaviscon Double Action Mint is used for the treatment of symptoms of gastro-oesophageal reflux such as acid regurgitation, heartburn and indigestion, which may occur, for example, following meals or during pregnancy and for symptoms of excess stomach acidity (hyperacidity).
page 1,
Before taking Gaviscon Double Action Mint Do not take this product if:
• You know you are allergic to any of the ingredients as very rarely difficulty in breathing and skin rashes have occurred (see further information for a full list of ingredients).
Take special care before treatment with Gaviscon Double Action Mint:
This medicine contains small amounts of sodium (11.06mmol per 20ml) and calcium (6.5 mmol per 20ml). If you have been advised to follow a diet restricted in either of these please consult your doctor.
You can take this product if you are pregnant or breast feeding.
This product contains methyl (E218) and propyl (E216) parahydroxybenzoates which may cause allergic reactions (possibly delayed).
Taking Other Medicines
Do not take this product within two hours of taking other medicines by mouth as it can interfere with the action of some other medicines.
This is especially important if you are taking antihistamines,
antibiotics (tetracyclines and quinolones such as norfloxacin), iron preparation, antifungals such as ketoconazole, digoxin and beta-blockers (for heart conditions), neuroleptics (for mental illness), thyroxine, chloroquine (for malaria) and bisphosphonates (for osteoporosis).
How to take Gaviscon Double Action Mint
Check that the cap seal is unbroken before first using this product. For oral use. Shake well before use.
Adults and children 12 years and over: Take 10-20ml (two to four 5ml spoonfuls) after meals and at bedtime, up to four times a day. Children under 12 years: Should only be taken on medical advice.
If you take too much of this product you may feel bloated. It is unlikely to cause you any harm, but please consult your doctor or pharmacist.
If you forget a dose it is not necessary to double the dose next time, just carry on taking as before.
Turn the leaflet for the further information
page 3i
After taking Gaviscon Double Action Mint:
If symptoms persist after 7 days consult your doctor Possible side effects
Very rarely (less than 1 in 10,000 patients treated) an allergic reaction to the ingredients may occur. Symptoms of this may include skin rash, itching, difficulty breathing, dizziness, or swelling of the face, lips, tongue or throat. If you experience these or any other side-effects stop taking the product and consult your doctor immediately.
Also you can help to make sure that medicines remain as safe as possible by reporting any unwanted side effects via the internet at http://www.mhra.gov.uk/yellowcard
Alternatively you can call Freephone 0808 100 3352 (available from 10 a.m. to 2 p.m. Mondays to Fridays) or fill in a paper form available from your local pharmacy.
page
How to store Gaviscon Double Action Mint
• Do not use this product after the expiry date (EXP: month/year) shown
• Use within 6 months of opening • Keep out of the reach and sight of children
• Do not store above 30°C. Do not refrigerate or freeze.
Further information
Each 10 ml of oral suspension contains 500mg sodium alginate, 213mg sodium bicarbonate and 325mg calcium carbonate as the active ingredients.
The other ingredients are carbomer, methyl (E218) and propyl (E216) parahydroxybenzoates, sodium saccharin, mint flavour, sodium hydroxide and water. This product does not contain sugar or gluten. Gaviscon Double Action Mint is available in bottles of 150ml, 300ml & 600ml.
Manufacturer and PL Holder in UK: Reckitt Benckiser Healthcare (UK) Ltd., Hull, HU8 7DS. Export distributors: Reckitt & Colman (Overseas) Ltd., Hull, HU8 7DS, UK.
Gaviscon and the sword and circle are trade marks.
Text Revised: February 2013
. _PaS? 5

CUSTOMER INFO: Minimum Point Size = 7.00pt
TBR1547-D8055386 - English
Each 10ml of oral suspension
contains s00"ig so^um algrate, 213mp ■ir'lium bice'bona*^ and 325mg calcium carko~ate. DOSAGE: For oral use. Shake well before ise. / 1i"s anr1 childr m 12 years and over: ~il )1' 20ml (two to four 5ml sp. jnfuls) after
Do not store above 30°C.
Do not refrigerate or freeze. KEEP OUT OF THE REACH AND SIGHT OF CHILDREN.
Use within 6 months of opening.
(GAVtSCON
Manufacturer and PL Holder in UK:
Reckitt Benckiser Healthcare (UK) Ltd., Hull, HU87DS.
150ml


PATIENT INFORMATION LEAFLET GAVTSCON DOUBLE ACTION MINT
Sodium alginate, Sodium bicarbonate, Calcium carbonate
Please read this leaflet carefully before you take this medicine. If you are not sure about anything ask your pharmacist or doctor.
What is Gaviscon Double Action Mint and what is it used for?
Gaviscon Double Action Mint is a combination of two antacids (calcium carbonate and sodium bicarbonate) and an alginate and works in two ways:
1) Neutralising excess stomach acid to relieve the pain and discomfort.
2) Forming a protective barrier over the stomach contents to soothe the burning pain in your chest.
Gaviscon Double Action Mint is used for the treatment of symptoms of gastro-oesophageal reflux such as acid regurgitation, heartburn and indigestion, which may occur, for example, following meals or during pregnancy and for symptoms of excess stomach acidity (hyperacidity).
page 1,
Before taking Gaviscon Double Action Mint Do not take this product if:
• You know you are allergic to any of the ingredients as very rarely difficulty in breathing and skin rashes have occurred (see further information for a full list of ingredients).
Take special care before treatment with Gaviscon Double Action Mint:
This medicine contains small amounts of sodium (11.06mmol per 20ml) and calcium (6.5 mmol per 20ml). If you have been advised to follow a diet restricted in either of these please consult your doctor.
You can take this product if you are pregnant or breast feeding.
This product contains methyl (E218) and propyl (E216) parahydroxybenzoates which may cause allergic reactions (possibly delayed).
Taking Other Medicines
Do not take this product within two hours of taking other medicines by mouth as it can interfere with the action of some other medicines.
This is especially important if you are taking antihistamines,
antibiotics (tetracyclines and quinolones such as norfloxacin), iron preparation, antifungals such as ketoconazole, digoxin and beta-blockers (for heart conditions), neuroleptics (for mental illness), thyroxine, chloroquine (for malaria) and bisphosphonates (for osteoporosis).
How to take Gaviscon Double Action Mint
Check that the cap seal is unbroken before first using this product. For oral use. Shake well before use.
Adults and children 12 years and over: Take 10-20ml (two to four 5ml spoonfuls) after meals and at bedtime, up to four times a day. Children under 12 years: Should only be taken on medical advice.
If you take too much of this product you may feel bloated. It is unlikely to cause you any harm, but please consult your doctor or pharmacist.
If you forget a dose it is not necessary to double the dose next time, just carry on taking as before.
Turn the leaflet for the further information
page 3i
After taking Gaviscon Double Action Mint:
If symptoms persist after 7 days consult your doctor Possible side effects
Very rarely (less than 1 in 10,000 patients treated) an allergic reaction to the ingredients may occur. Symptoms of this may include skin rash, itching, difficulty breathing, dizziness, or swelling of the face, lips, tongue or throat. If you experience these or any other side-effects stop taking the product and consult your doctor immediately.
Also you can help to make sure that medicines remain as safe as possible by reporting any unwanted side effects via the internet at http://www.mhra.gov.uk/yellowcard
Alternatively you can call Freephone 0808 100 3352 (available from 10 a.m. to 2 p.m. Mondays to Fridays) or fill in a paper form available from your local pharmacy.
page
How to store Gaviscon Double Action Mint
• Do not use this product after the expiry date (EXP: month/year) shown
• Use within 6 months of opening • Keep out of the reach and sight of children
• Do not store above 30°C. Do not refrigerate or freeze.
Further information
Each 10 ml of oral suspension contains 500mg sodium alginate, 213mg sodium bicarbonate and 325mg calcium carbonate as the active ingredients.
The other ingredients are carbomer, methyl (E218) and propyl (E216) parahydroxybenzoates, sodium saccharin, mint flavour, sodium hydroxide and water. This product does not contain sugar or gluten. Gaviscon Double Action Mint is available in bottles of 150ml, 300ml & 600ml.
Manufacturer and PL Holder in UK: Reckitt Benckiser Healthcare (UK) Ltd., Hull, HU8 7DS. Export distributors: Reckitt & Colman (Overseas) Ltd., Hull, HU8 7DS, UK.
Gaviscon and the sword and circle are trade marks.
Text Revised: February 2013
. _PaS? 5

CUSTOMER INFO: Minimum Point Size = 7.00pt
TBR1547-D8055386 - English
"j
w
3
3
o
o
to
to
LJ
S'
PRINTING READS This Way
PATIENT INFORMATION LEAFLET GAVISCON DOUBLE ACTION MINT Sodium alginate Sodium bicarbonate Calcium carbonate
Please read this leaflet carefully before you take this medicine. If you are not sure about anything ask your pharmacist or doctor. What is Gaviscon Double Action Mint and
what is it used for?
Gaviscon Double Action Mint is a combination of two antacids (calcium carbonate and sodium bicarbonate) and an alginate and works in two ways:
1) Neutralising excess stomach acid to relieve the pain and discomfort.
2) Forming a protective barrier over the stomach contents to soothe the burning pain in your chest.
Gaviscon Double Action Mint is used for the treatment of symptoms ofjastto-qerophageal
reflux such as acid regurgitation, heartburn and indigestion, which may occur, for example, following meals or during pregnancy and for symptoms of excess stomach acidity (hyperacidity).
Before taking Gaviscon Double Action
Mint
Do not take this product if:
• You know you are allergic to any of the ingredients as very rarely difficulty in breathing and skin rashes have occurred (see further information for a full list of ingredients).
Take special care before treatment with Gaviscon Double Action Mint:
This medicine contains small amounts of sodium (11.06mmol per 20ml) and calcium (6.5 mmol per 20ml). If you have been advised to follow a diet restricted in either of these please consult your doctor.
You can take this product if you are pregiant
or breast feeding.
This product contains methyl (E218) and propyl (E216) parahydroxybenzoates which may cause allergic reactions (possibly delayed).
Taking Other Medicines
Do not take this product within two hours of taking other medicines by mouth as it can interfere with the action of some other medicines. This is especially important if you are taking antihistamines, antibiotics (tetracyclines and quinolones such as norfloxacin), iron preparation, antifungals such as ketoconazole, digoxin and beta-blockers (for heart conditions), neuroleptics (for mental illness), thyroxine, chloroquine (for malaria) and bisphosphonates (for osteoporosis).
_8085162_
How to take Gaviscon Double Action Mint
Check that the cap seal is unbroken before first using this product.
For oral use. Shake well before use.
Adults and children 12 years and over: Take 10-20ml (two to four 5ml spoonfuls) after meals and at bedtime, up to four times a day. Children under 12 years: Should only be taken on medical advice. If you take too much of this product you may feel bloated. It is unlikely to cause you any harm, but please consult your doctor or pharmacist. If you forget a dose it is not necessary to double the dose next time, just cany on taking as before. After taking Gaviscon Double Action Mint:
If symptoms persist after 7 days consult your doctor.
Possible side effects
Very rarely (less than 1 in 10,000 patients treated) an allergic reaction to the ingredients
may occur. Symptoms of this may include skin rash, itching, difficulty breathing, dizziness, or swelling of the face, lips, tongue or throat. If you experience these or any other side-effects stop taking the product and consult your doctor immediately. Also you can help to make sure that medicines remain as safe as possible by reporting any unwanted side effects via the internet at http://www.mhra.gov.uk/yellowcard Alternatively you can call Freephone 0808 100 3352 (available from 10 a.m. to 2 p.m. Mondays to Fridays) or fill in a paper form available from your local pharmacy. How to store Gaviscon Double Action Mint
Do not use this product after the expiry date (EXP: month/year) shown
• Use within 6 months of opening
• Keep out of the reach and sight of children
• Do not store above 30°C •Do not reffigerate or freeze
Further information
Each 10 ml of oral suspension contains 500mg sodium alginate, 213mg sodium bicarbonate and 325mg calcium carbonate as the active ingedients.
The other ingedients are carbomer, methyl (E218) and propyl (E216) parahydroxybenzoates, sodium saccharin, mint flavour, sodium hydroxide and water. This product does not contain sugar or gluten. Gaviscon Double Action Mint is available in 150ml, 300ml & 600ml bottles.
Manufacturer and PL Holder in UK:
Reckitt Benckiser Healthcare (UK) Ltd.,
Hull, HU8 7DS.
Gaviscon and the sword and circle are trade marks
Text Revised: February 2013
8085162!
|
TBR1291- |
D0006047 | |||||||||
|
m |
a |
m |
■ | |||||||
|
io | ||||||||||
|
ffl | ||||||||||
|
m | ||||||||||
CUSTOMER INFO: Minimum Point Size = 7.00pt Leaflet = 9 pt Label = 7pt
"j
w
3
3
o
o
to
to
LJ
S'
PRINTING READS This Way
PATIENT INFORMATION LEAFLET GAVISCON DOUBLE ACTION MINT Sodium alginate Sodium bicarbonate Calcium carbonate
Please read this leaflet carefully before you take this medicine. If you are not sure about anything ask your pharmacist or doctor. What is Gaviscon Double Action Mint and
what is it used for?
Gaviscon Double Action Mint is a combination of two antacids (calcium carbonate and sodium bicarbonate) and an alginate and works in two ways:
1) Neutralising excess stomach acid to relieve the pain and discomfort.
2) Forming a protective barrier over the stomach contents to soothe the burning pain in your chest.
Gaviscon Double Action Mint is used for the treatment of symptoms ofjastto-qerophageal
reflux such as acid regurgitation, heartburn and indigestion, which may occur, for example, following meals or during pregnancy and for symptoms of excess stomach acidity (hyperacidity).
Before taking Gaviscon Double Action
Mint
Do not take this product if:
• You know you are allergic to any of the ingredients as very rarely difficulty in breathing and skin rashes have occurred (see further information for a full list of ingredients).
Take special care before treatment with Gaviscon Double Action Mint:
This medicine contains small amounts of sodium (11.06mmol per 20ml) and calcium (6.5 mmol per 20ml). If you have been advised to follow a diet restricted in either of these please consult your doctor.
You can take this product if you are pregiant
or breast feeding.
This product contains methyl (E218) and propyl (E216) parahydroxybenzoates which may cause allergic reactions (possibly delayed).
Taking Other Medicines
Do not take this product within two hours of taking other medicines by mouth as it can interfere with the action of some other medicines. This is especially important if you are taking antihistamines, antibiotics (tetracyclines and quinolones such as norfloxacin), iron preparation, antifungals such as ketoconazole, digoxin and beta-blockers (for heart conditions), neuroleptics (for mental illness), thyroxine, chloroquine (for malaria) and bisphosphonates (for osteoporosis).
_8085162_
How to take Gaviscon Double Action Mint
Check that the cap seal is unbroken before first using this product.
For oral use. Shake well before use.
Adults and children 12 years and over: Take 10-20ml (two to four 5ml spoonfuls) after meals and at bedtime, up to four times a day. Children under 12 years: Should only be taken on medical advice. If you take too much of this product you may feel bloated. It is unlikely to cause you any harm, but please consult your doctor or pharmacist. If you forget a dose it is not necessary to double the dose next time, just cany on taking as before. After taking Gaviscon Double Action Mint:
If symptoms persist after 7 days consult your doctor.
Possible side effects
Very rarely (less than 1 in 10,000 patients treated) an allergic reaction to the ingredients
may occur. Symptoms of this may include skin rash, itching, difficulty breathing, dizziness, or swelling of the face, lips, tongue or throat. If you experience these or any other side-effects stop taking the product and consult your doctor immediately. Also you can help to make sure that medicines remain as safe as possible by reporting any unwanted side effects via the internet at http://www.mhra.gov.uk/yellowcard Alternatively you can call Freephone 0808 100 3352 (available from 10 a.m. to 2 p.m. Mondays to Fridays) or fill in a paper form available from your local pharmacy. How to store Gaviscon Double Action Mint
Do not use this product after the expiry date (EXP: month/year) shown
• Use within 6 months of opening
• Keep out of the reach and sight of children
• Do not store above 30°C •Do not reffigerate or freeze
Further information
Each 10 ml of oral suspension contains 500mg sodium alginate, 213mg sodium bicarbonate and 325mg calcium carbonate as the active ingedients.
The other ingedients are carbomer, methyl (E218) and propyl (E216) parahydroxybenzoates, sodium saccharin, mint flavour, sodium hydroxide and water. This product does not contain sugar or gluten. Gaviscon Double Action Mint is available in 150ml, 300ml & 600ml bottles.
Manufacturer and PL Holder in UK:
Reckitt Benckiser Healthcare (UK) Ltd.,
Hull, HU8 7DS.
Gaviscon and the sword and circle are trade marks
Text Revised: February 2013
8085162!
|
TBR1291- |
D0006047 | |||||||||
|
m |
a |
m |
■ | |||||||
|
io | ||||||||||
|
ffl | ||||||||||
|
m | ||||||||||
CUSTOMER INFO: Minimum Point Size = 7.00pt Leaflet = 9 pt Label = 7pt
WHAT IS THIS PRODUCT? Each 10ml of oral suspension contains 500mg sodium alginate, 213mg sodium bicarbonate and 325mg calcium carbonate.
e500ml
For oral use.
Shake well before use.
If symptoms persist after 7 days consult your doctor.
Contains sodium, calcium, methyl (E218) and propyl (E216) parahydroxybenzoates.
See leaflet for further information.
Do not store above 30°C. Do not refrigerate or freeze. Use within 6 months of opening.
KEEP OUT OF THE REACH AND SIGHT OF CHILDREN.
Manufacturer and PL Holder in UK: Reckitt Benckiser Healthcare (UK) Ltd., Hull, HU87DS.
PL00063/0552
Peel here. Do not remove.
Gaviscon a cm on fCJL
Mint jfk^S
Oral suspension ■ ■
Sodium alginate -
Sodium bicarbonate Calcium carbonate
\ DISPENSING PACK.
NOT FOR RETAIL SALE
isasizDsasiffla
PATIENT INFORMATION LEAFLET GAVISCON DOUBLE ACTION MINT
Sodium alginate Sodium bicarbonate Calcium carbonate
Please read this leaflet carefully before you take this medicine. If you are not sure about anything ask your pharmacist or doctor. What is Gaviscon Double Action Mint and
regurgitation, heartburn and indigestion, winch may occur, for example, following meals or during pregnancy and for symptoms of excess stomach acidity (hyperacidity).
Before taking Gaviscon Double Action
what is it used for?
Gaviscon Double Action Mint is a combination of two antacids (calcium carbonate and sodium bicarbonate) and an alginate and works in two ways:
1) Neutralising excess stomach acid to relieve the pain and discomfort.
2) Forming a protective barrier over the stomach contents to soothe the burning pain in your chest.
Gaviscon Double Action Mint is used for the treatment of symptoms of gastro-oesophageal reflux such as acid
Mint
Do not take this product if:
•You know you are allergic to any of the ingredients as very rarely difficulty in breathing and skin rashes have occurred (see further information for a full list of ingredients).
Take special care before treatment with Gaviscon Double Action Mint:
This medicine contains small amounts of sodium (11.06mmol per 20ml) and calcium (6.5 mmol per 20ml). If you have been advised to follow a diet restricted in either of these please consult your doctor.
You can take this product if you are pregnant or breast feeding.
This product contains methyl (E218) and propyl (E216) parahydroxybenzoates which may cause allergic reactions (possibly delayed).
Taking Other Medicines
Do not take this product within two hours of taking other medicines by mouth as it can interfere with the action of some other medicines. This is especially important if you are taking antihistamines, antibiotics (tetracyclines and quinolones such as norfloxacin), iron preparation, antifungals such as ketoconazole, digoxin and beta-blockers (for heart conditions), neuroleptics (for mental illness), thyroxine, chloroquine (for malaria) and biphosphonates (for osteoporosis).
How to take Gaviscon Double Action Mint
Check that the cap seal is unbroken before first using this product.
For oral use. Shake well before use.
RB75645

|
Adults and children 12 years and over: Take 10-20ml (two to four 5ml spoonfuls) after meals and at bedtime, up to four times a day, or as directed by your doctor. Children under 12 years: Should only be taken on medical advice. If you take too much of this product you may feel bloated. It is unlikely to cause you any harm, but please consult your doctor or pharmacist. If you forget a dose it is not necessary to double the dose |
and consult your doctor immediately. Also you can help to make sure that medicines remain as safe as possible by reporting any unwanted side effects via the internet at http ://www.mhra.gov.uk/yellowcard Alternatively you can call Freephone 0808 100 3352 (available from 10 a.m. to 2 p.m. Mondays to Fridays) or fill in a paper form available from your local pharmacy. How to store Gaviscon Double Action Mint |
(E218) and propyl (E216) parahydroxybenzoates, sodium saccharin, mint flavour, sodium hydroxide and water. This product does not contain sugar or gluten. Manufacturer and PL Holder in UK: Reckitt Benckiser Healthcare (UK) Ltd., Hull, HU8 7DS. Export distributors: Reckitt & Colman (Overseas) Ltd., Hull, HU8 7DS, UK. Gaviscon and the sword and circle are trade marks. Text Revised: July 2015 |
|
next time, just carry on taking as before. After taking Gaviscon Double Action Mint: If symptoms persist after 7 days consult your doctor. Possible side effects |
Do not use this product after the expiry date (EXP: month/year) shown • Use within 6 months of opening • Keep out of the reach and sight of children • Do not store above 30°C. | |
|
Very rarely (less than 1 in 10,000 patients treated) an allergic reaction to the ingredients |
Do not refrigerate or freeze Further information | |
|
may occur. Symptoms of this may include skin rash, itching, difficulty breathing, dizziness, or swelling of the face, lips, tongue or throat. If you experience these or any other side-effects stop taking the product |
Each 10 ml of oral suspension contains 500mg sodium alginate, 213mg sodium bicarbonate and 325mg calcium carbonate as the active ingredients. The other ingredients are carbomer, methyl |
RB75645 |
WHAT IS THIS PRODUCT? Each 10ml of oral suspension contains 500mg sodium alginate, 213mq ionium oicarbonatc a.iu 3Lamg calcium carbonate.
e500ml
For oral use.
Shake weil before use.
If symptoiiis persist aftei 7 days consult your doctor.
Contains c odium, c l ium, methyl (E218) and propyl (E216) parahydroxybenzoates.
See leaflet for further information.
Do not store above 30°C. Do not refrigerate or freeze. Use within 6 months of opening.
KEEP OUT OF THE REACH AND SIGHT OF CHILDREN.
Manufacturer and PL Holder in UK: Reckitt Benckiser Healthcare (UK) Ltd., Hull, HU87DS.
PL00063/0552
Peel here. Do not remove.
GAVISCON
Mint jfk ^9^
Oral suspension ■ 1 '
Sodium alginate Sodium bicarbonate Calcium carbonate
\ DISPENSING PACK. 1 NOT FOR RETAIL SALE
iBDBiZjSiSffiBi
PATIENT INFORMATION LEAFLET GAVISCON DOUBLE ACTION MINT
Sodium alginate Sodium bicarbonate Calcium carbonate
Please read this leaflet carefully before you take this medicine. If you are not sure about anything ask your pharmacist or doctor. What is Gaviscon Double Action Mint and
regurgitation, heartburn and indigestion, winch may occur, for example, following meals or during pregnancy and for symptoms of excess stomach acidity (hyperacidity).
Before taking Gaviscon Double Action
what is it used for?
Gaviscon Double Action Mint is a combination of two antacids (calcium carbonate and sodium bicarbonate) and an alginate and works in two ways:
1) Neutralising excess stomach acid to relieve the pain and discomfort.
2) Forming a protective barrier over the stomach contents to soothe the burning pain in your chest.
Gaviscon Double Action Mint is used for the treatment of symptoms of gastro-oesophageal reflux such as acid
Mint
Do not take this product if:
•You know you are allergic to any of the ingredients as very rarely difficulty in breathing and skin rashes have occurred (see further information for a full list of ingredients).
Take special care before treatment with Gaviscon Double Action Mint:
This medicine contains small amounts of sodium (11.06mmol per 20ml) and calcium (6.5 mmol per 20ml). If you have been advised to follow a diet restricted in either of these please consult your doctor.
You can take this product if you are pregnant or breast feeding.
This product contains methyl (E218) and propyl (E216) parahydroxybenzoates which may cause allergic reactions (possibly delayed).
Taking Other Medicines
Do not take this product within two hours of taking other medicines by mouth as it can interfere with the action of some other medicines. This is especially important if you are taking antihistamines, antibiotics (tetracyclines and quinolones such as norfloxacin), iron preparation, antifungals such as ketoconazole, digoxin and beta-blockers (for heart conditions), neuroleptics (for mental illness), thyroxine, chloroquine (for malaria) and biphosphonates (for osteoporosis).
How to take Gaviscon Double Action Mint
Check that the cap seal is unbroken before first using this product.
For oral use. Shake well before use.
RB75645

|
Adults and children 12 years and over: Take 10-20ml (two to four 5ml spoonfuls) after meals and at bedtime, up to four times a day, or as directed by your doctor. Children under 12 years: Should only be taken on medical advice. If you take too much of this product you may feel bloated. It is unlikely to cause you any harm, but please consult your doctor or pharmacist. If you forget a dose it is not necessary to double the dose |
and consult your doctor immediately. Also you can help to make sure that medicines remain as safe as possible by reporting any unwanted side effects via the internet at http ://www.mhra.gov.uk/yellowcard Alternatively you can call Freephone 0808 100 3352 (available from 10 a.m. to 2 p.m. Mondays to Fridays) or fill in a paper form available from your local pharmacy. How to store Gaviscon Double Action Mint |
(E218) and propyl (E216) parahydroxybenzoates, sodium saccharin, mint flavour, sodium hydroxide and water. This product does not contain sugar or gluten. Manufacturer and PL Holder in UK: Reckitt Benckiser Healthcare (UK) Ltd., Hull, HU8 7DS. Export distributors: Reckitt & Colman (Overseas) Ltd., Hull, HU8 7DS, UK. Gaviscon and the sword and circle are trade marks. Text Revised: July 2015 |
|
next time, just carry on taking as before. After taking Gaviscon Double Action Mint: If symptoms persist after 7 days consult your doctor. Possible side effects |
Do not use this product after the expiry date (EXP: month/year) shown • Use within 6 months of opening • Keep out of the reach and sight of children • Do not store above 30°C. | |
|
Very rarely (less than 1 in 10,000 patients treated) an allergic reaction to the ingredients |
Do not refrigerate or freeze Further information | |
|
may occur. Symptoms of this may include skin rash, itching, difficulty breathing, dizziness, or swelling of the face, lips, tongue or throat. If you experience these or any other side-effects stop taking the product |
Each 10 ml of oral suspension contains 500mg sodium alginate, 213mg sodium bicarbonate and 325mg calcium carbonate as the active ingredients. The other ingredients are carbomer, methyl |
RB75645 |

|
• • |
• |
• |
• |
• |
• • |
• |
• • | |||
|
• • |
• |
• |
• |
• |
• | |||||
|
• • |
• |
• |
• | |||||||
|
• • |
• |
• |
• |
• |
• |
• |
• • • |
• • |
• • | |
|
• |
• |
• |
• |
• |
• • |
• • |
• | |||
|
• |
• • |
• |
• |
• |
• | |||||
|
• • |
• |
• • |
• | |||||||
|
• |
• |
• • | ||||||||
|
• |
• |
• |
WHAT IS THIS PRODUCT? Each 10ml of oral suspension contains 500mg sodium alginate, 213mg sodium bicarbonate and 325mg calcium carbonate. DOSAGE: For oral use. Shake well before use.
Adults and children 12years and over: Take 10-20ml (two to four 5ml spoonfuls) after meals and at bedtime, up to four times a day.
Children under 12 years: Should only be taken on medical advice.
If symptoms persist after 7 days consult your doctor. Contains sodium, calcium, methyl (E218) and propyl (E216) parahydroxybenzoates.
See leaflet for further information.
Manufacturer and PL Holder in UK: Reckitt Benckiser Healthcare (UK) Ltd., Hull, HU87DS.
PL00063/0552
Do not store above 30°C. Do not refrigerate or freeze. Use within 6 months of opening.
Mint
Oral suspension Sodium alginate Sodium bicarbonate Calcium carbonate
0
Peel here. Do not remove.
Heartburn & Indigestion Fast Effective Relief Lasts up to 4 hours

PATIENT INFORMATION LEAFLET GAVISCON DOUBLE ACTION MINT
Sodium alginate Sodium bicarbonate Calcium carbonate
Please read this leaflet carefully before you take this medicine. If you are not sure about anything ask your pharmacist or doctor. What is Gaviscnn Double Action Mint and
regurgitation, heartburn and indigestion, which may occur, for example, following meals or during pregnancy and for symptoms of excess stomach acidity (hyperacidity).
Before taking Gaviscon Double Action
what is it used for?
Gaviscon Double Action Mint is a combination of two antacids (calcium carbonate and sodium bicarbonate) and an alginate and works in two ways:
1) Neutralising excess stomach acid to relieve the pain and discomfort.
2) Forming a protective barrier over the stomach contents to soothe the burning pain in your chest.
Gaviscon Double Action Mint is used for the treatment of symptoms of gastro-oesophageal reflux such as acid
Mint
Do not take this product if:
• You know you are allergic to any of the ingredients as very rarely difficulty in breathing and skin rashes have occurred (see further information for a Ml list of ingredients).
Take special care before treatment with Gaviscon Double Action Mint:
This medicine contains small amounts of sodium (11.06mmol per 20ml) and calcium (6.5 mmol per 20ml). If you have been advised to follow a diet restricted in either of these please consult your doctor.
You can take this product if you are pregnant or breast feeding.
This product contains methyl (E218) and propyl (E216) parahydroxybenzoates which may cause allergic reactions (possibly delayed).
Taking Other Medicines
Do not take this product within two hours of taking other medicines by mouth as it can interfere with the action of some other medicines. This is especially important if you are taking antihistamines, antibiotics (tetracyclines and quinolones such as norfloxacin), iron preparation, antifungals such as ketoconazole, digoxin and beta-blockers (for heart conditions), neuroleptics (for mental illness), thyroxine, chloroquine (for malaria) and bisphosphonates (for osteoporosis).
How to take Gaviscnn Double Action Mint
Check that the cap seal is unbroken before first using this product.
For oral use. Shake well before use.
8085163

TBR1290-D0014922
Adults and children 12 years and over:
Take 10-20ml (two to four 5ml spoonMs) after meals and at bedtime, up to four times a day. Children under 12 years: Should only be taken on medical advice. If you take too much of this product you may feel bloated. It is unlikely to cause you any harm, but please consult your doctor or pharmacist. If you forget a dose it is not necessary to double the dose next time, just carry on taking as before. After taking Gaviscon Double Action Mint:
If symptoms persist after 7 days consult your doctor.
Possible side effects
Very rarely (less than 1 in 10,000 patients treated) an allergic reaction to the ingredients may occur. Symptoms of this may include skin rash, itching, difficulty breathing, dizziness, or swelling of the face, lips, tongue or throat. If you experience these or any other side-effects stop taking the product
and consult your doctor immediately. Also you can help to make sure that medicines remain as safe as possible by reporting any unwanted side effects via the internet at http://www.mhra.gov.uk/yellowcard Alternatively you can call Freephone 0808 100 3352 (available from 10 a.m. to 2 p.m. Mondays to Fridays) or fill in a paper form available from your local pharmacy. How to store Gaviscon Double Action
Do not use this product after the expiry date (EXP: month/year) shown
• Use within 6 months of opening
• Keep out of the reach and sight of children
• Do not store above 30°C.
Do not refrigerate or freeze Further information
Each 10 ml of oral suspension contains 500mg sodium alginate, 213mg sodium bicarbonate and 325mg calcium carbonate as the active ingredients.
The other ingredients are carbomer, methyl
(E218) and propyl (E216) parahydroxybenzoates, sodium saccharin, mint flavour, sodium hydroxide and water. This product does not contain sugar or gluten. Gaviscon Double Action Mint is available in bottles of 150ml, 300ml & 600ml
Manufacturer and PL Holder in UK:
Reckitt Benckiser Healthcare (UK) Ltd., Hull, HU8 7DS.
Export distributors: Reckitt & Colman (Overseas) Ltd., Hull, HU8 7DS, UK. Gaviscon and the sword and circle are trade marks.
Text Revised: February 2013
8085163'

CUSTOMER 11MFO: Minimum Point Size = 7.00pt Leaflet = 9 pt Label = 7pt
WHAT IS THIS PRODUCT? Each 10ml of oral suspension contains 500mg sodium alginate, 213mg sodiu.i Lic^rbor.jte and 325r. g i_aUiL.ii ca.innate. DOSAjL: For ori-i use. Sha..e well befor„ use.
Adults and child.flu 12veaid and ovei.Takb 10-20ml (two to four 5ml spoonfuls) after meals and at bedtime up t" four times a day.
Children urder P '-ears: Should only be taken on medical advice.
If syrr at ams ; 3r 5r‘ afte 7 days consult your doctor. Contains SLdium, tjli!u..i, methyl (E218) and propyl (E216) parahydrLxybe.,zoates.
See leaflet for further information.
Manufacturer and PL Holder in UK: Reckitt Benckiser Healthcare (UK) Ltd., Hull, HU87DS.
PL00063/0552
Do not store above 30°C. Do not refrigerate or freeze. Use within 6 months of opening.
Mint
Oral suspension Sodium alginate Sodium bicarbonate Calcium carbonate
0
Peel here. Do not remove.
Heartburn & Indigestion Fast Effective Relief Lasts up to 4 hours

PATIENT INFORMATION LEAFLET GAVISCON DOUBLE ACTION MINT
Sodium alginate Sodium bicarbonate Calcium carbonate
Please read this leaflet carefully before you take this medicine. If you are not sure about anything ask your pharmacist or doctor. What is Gaviscnn Double Action Mint and
regurgitation, heartburn and indigestion, which may occur, for example, following meals or during pregnancy and for symptoms of excess stomach acidity (hyperacidity).
Before taking Gaviscon Double Action
what is it used for?
Gaviscon Double Action Mint is a combination of two antacids (calcium carbonate and sodium bicarbonate) and an alginate and works in two ways:
1) Neutralising excess stomach acid to relieve the pain and discomfort.
2) Forming a protective barrier over the stomach contents to soothe the burning pain in your chest.
Gaviscon Double Action Mint is used for the treatment of symptoms of gastro-oesophageal reflux such as acid
Mint
Do not take this product if:
• You know you are allergic to any of the ingredients as very rarely difficulty in breathing and skin rashes have occurred (see further information for a Ml list of ingredients).
Take special care before treatment with Gaviscon Double Action Mint:
This medicine contains small amounts of sodium (11.06mmol per 20ml) and calcium (6.5 mmol per 20ml). If you have been advised to follow a diet restricted in either of these please consult your doctor.
You can take this product if you are pregnant or breast feeding.
This product contains methyl (E218) and propyl (E216) parahydroxybenzoates which may cause allergic reactions (possibly delayed).
Taking Other Medicines
Do not take this product within two hours of taking other medicines by mouth as it can interfere with the action of some other medicines. This is especially important if you are taking antihistamines, antibiotics (tetracyclines and quinolones such as norfloxacin), iron preparation, antifungals such as ketoconazole, digoxin and beta-blockers (for heart conditions), neuroleptics (for mental illness), thyroxine, chloroquine (for malaria) and bisphosphonates (for osteoporosis).
How to take Gaviscnn Double Action Mint
Check that the cap seal is unbroken before first using this product.
For oral use. Shake well before use.
8085163

TBR1290-D0014922
Adults and children 12 years and over:
Take 10-20ml (two to four 5ml spoonMs) after meals and at bedtime, up to four times a day. Children under 12 years: Should only be taken on medical advice. If you take too much of this product you may feel bloated. It is unlikely to cause you any harm, but please consult your doctor or pharmacist. If you forget a dose it is not necessary to double the dose next time, just carry on taking as before. After taking Gaviscon Double Action Mint:
If symptoms persist after 7 days consult your doctor.
Possible side effects
Very rarely (less than 1 in 10,000 patients treated) an allergic reaction to the ingredients may occur. Symptoms of this may include skin rash, itching, difficulty breathing, dizziness, or swelling of the face, lips, tongue or throat. If you experience these or any other side-effects stop taking the product
and consult your doctor immediately. Also you can help to make sure that medicines remain as safe as possible by reporting any unwanted side effects via the internet at http://www.mhra.gov.uk/yellowcard Alternatively you can call Freephone 0808 100 3352 (available from 10 a.m. to 2 p.m. Mondays to Fridays) or fill in a paper form available from your local pharmacy. How to store Gaviscon Double Action
Do not use this product after the expiry date (EXP: month/year) shown
• Use within 6 months of opening
• Keep out of the reach and sight of children
• Do not store above 30°C.
Do not refrigerate or freeze Further information
Each 10 ml of oral suspension contains 500mg sodium alginate, 213mg sodium bicarbonate and 325mg calcium carbonate as the active ingredients.
The other ingredients are carbomer, methyl
(E218) and propyl (E216) parahydroxybenzoates, sodium saccharin, mint flavour, sodium hydroxide and water. This product does not contain sugar or gluten. Gaviscon Double Action Mint is available in bottles of 150ml, 300ml & 600ml
Manufacturer and PL Holder in UK:
Reckitt Benckiser Healthcare (UK) Ltd., Hull, HU8 7DS.
Export distributors: Reckitt & Colman (Overseas) Ltd., Hull, HU8 7DS, UK. Gaviscon and the sword and circle are trade marks.
Text Revised: February 2013
8085163'

CUSTOMER 11MFO: Minimum Point Size = 7.00pt Leaflet = 9 pt Label = 7pt