Gedarel Ed 20 Micrograms/150 Micrograms -28 Film-Coated Tablet
Package Leaflet: Information for the user
20 micrograms/150 micrograms -28 film-coated tablets
GEDAREL® ED
ethinylestradiol/desogestrel
Important things to know about combined hormonal contraceptives (CHCs):
• They are one of the most reliable reversible methods of contraception if used correctly.
• They slightly increase the risk of having a blood clot in the veins and arteries, especially in the first year or when restarting a combined hormonal contraceptive following a break of 4 or more weeks.
• Please be alert and see your doctor if you think you may have symptoms of a blood clot (see section 2 "Blood clots”).
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them even if their signs of illness are the same as yours.
- If you get any side effects talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
The name of your medicine is 'Gedarel ED 20 micrograms/150 micrograms -28 film-coated tablets', but will be referred to as 'Gedarel ED -28' throughout the remainder of this leaflet.
1. What Gedarel ED -28 is and what it is used for
2. What you need to know before you take Gedarel ED -28
3. How to take Gedarel ED -28
4. Possible side effects
5. How to store Gedarel ED -28
6. Contents of the pack and other information
1. What Gedarel ED -28 is and what it is used for
Gedarel ED -28 is a contraceptive pill and is used to prevent pregnancy.
Each slightly yellow film-coated tablet contains a small amount of two different female hormones, namely desogestrel and ethinylestradiol. The green film-coated tablets contain no active substances and are also called placebo tablets.
Contraceptive pills that contain two hormones are called "combination” pills.
The combined contraceptive pill protects you against getting pregnant in three ways. These hormones
1. stop the ovary from releasing an egg each month (ovulation).
2. also thicken the fluid (at the neck of the womb making it more difficult for the sperm to reach the egg.
3. alter the lining of the womb to make it less likely to accept a fertilised egg.
General information
If taken correctly, the pill is an effective reversible form of contraception. However, in certain circumstances the effectiveness of the pill may reduce or you should stop taking the pill (see later). In these cases either do not have sex, or use extra contraceptive precautions (such as condoms or spermicides) during intercourse to ensure effective contraception.
Do not use rhythm or temperature methods. These methods can be unreliable because Gedarel ED -28 alters the monthly changes of body temperature and of cervical mucus.
Remember, combined oral contraceptive pills like Gedarel ED -28 will not protect you against sexually-transmitted diseases (such as AIDS). Only condoms can help to do this.
2. What you need to know before you take Gedarel ED -28 General notes
Before you start using Gedarel ED -28 you should read the information on blood clots in section 2. It is particularly important to read the symptoms of a blood clot - see section 2 "Blood clots”.
Before you can begin taking Gedarel ED -28, your doctor will ask you some questions about your personal health history and that of your close relatives. The doctor will also measure your blood pressure, and depending upon your personal situation, may also carry out some other tests.
Do not take Gedarel ED -28
You should not use Gedarel ED -28 if you have any of the conditions listed below. If you do have any of the conditions listed below, you must tell your doctor. Your doctor will discuss with you what other form of birth control would be more appropriate
- if you have (or have ever had) a blood clot in a blood vessel of your legs (deep vein thrombosis, DVT), your lungs (pulmonary embolus, PE) or other organs;
- if you know you have a disorder affecting your blood clotting - for instance, protein C deficiency, protein S deficiency, antithrombin-III deficiency, Factor V Leiden or antiphospholipid antibodies;
- if you need an operation or if you are off your feet for a long time (see section 'Blood clots');
- if you have ever had a heart attack or a stroke;
- if you have (or have ever had) angina pectoris (a condition that causes severe chest pain and may be a first sign of a heart attack) or transient ischaemic attack [TIA - temporary stroke symptoms];
- if you have any of the following diseases that may increase your risk of a clot in the arteries:
- severe diabetes with blood vessel damage
- very high blood pressure
- a very high level of fat in the blood (cholesterol or triglycerides)
- a condition known as hyperhomocysteinaemia;
- if you have (or have ever had) a type of migraine called 'migraine with aura';
- if you have (had) an inflammation of the pancreas (pancreatitis);
- if you have or have had in the past a liver disease and your liver function is still not normal;
- if you have or have had a tumour in the liver;
- if you have (had) or if you are suspected of having breast cancer or cancer of the genital organs;
- if you have any unexplained bleeding from the vagina;
- if you have endometrial hyperplasia (excessive proliferation of the cells of the inner lining of the womb);
- if you are pregnant or think you might be;
- if you are allergic to ethinylestradiol or desogestrel, or any of the other ingredients of this medicine (listed in section 6). This can be recognised by itching, rash or swelling.
Warnings and precautions
Talk to your doctor or pharmacist before taking Gedarel ED -28.
When should you contact your doctor?
Seek urgent medical attention
- if you notice possible signs of a blood clot that may mean you are suffering from a blood clot in the leg (i.e. deep vein thrombosis), a blood clot in the lung (i.e. pulmonary embolism), a heart attack or a stroke (see 'Blood clots' (thrombosis) section below).
For a description of the symptoms of these serious side effects please go to "How to recognise a blood clot”._
In some situations you need to take special care while using Gedarel ED -28 or any other combined hormonal contraceptive, and it may be necessary that you are regularly checked by your doctor.
Tell your doctor if any of the following conditions apply to you.
If the condition develops, or gets worse while you are using Gedarel ED -28, you should also tell your doctor
- if you have Crohn's disease or ulcerative colitis (chronic inflammatory bowel disease);
- if you have systemic lupus erythematosus (SLE - a disease affecting your natural defence system);
- if you have haemolytic uraemic syndrome (HUS - a disorder of blood clotting causing failure of the kidneys);
- if you have sickle cell anaemia (an inherited disease of the red blood cells);
- if you have elevated levels of fat in the blood (hypertriglyceridaemia) or a positive family history for this condition. Hypertriglyceridaemia has been associated with an increased risk of developing pancreatitis (inflammation of the pancreas).
- if you need an operation, or you are off your feet for a long time (see in section 2 'Blood clots');
- if you have just given birth you are at an increased risk of blood clots. You should ask your doctor how soon after delivery you can start taking Gedarel ED -28.
- if you have an inflammation in the veins under the skin (superficial thrombophlebitis);
- if you have varicose veins;
- if a close relative has or has had breast cancer;
- if you have a disease of the liver or the gallbladder;
- if you have diabetes;
- if you have depression;
- if you have epilepsy (see "Other medicines and Gedarel ED -28”);
- if you have a disease that first appeared during pregnancy or earlier use of sex hormones (for example, hearing loss, porphyria (a disease of the blood), gestational herpes (skin rash with vesicles during pregnancy), Sydenham's chorea (a disease of the nerves in which sudden movements of the body occur);
- if you have or have ever had chloasma (golden brown pigment patches, so called "pregnancy patches”, especially on the face). If this is the case, avoid direct exposure to sunlight or ultraviolet light.
- if you have hereditary angioedema, products containing estrogens may induce or worsen symptoms of angioedema. You should see your doctor immediately if you experience symptoms of angioedema such as swollen face, tongue and/or pharynx and/or difficulty swallowing or hives together with difficulty breathing.
BLOOD CLOTS
Using a combined hormonal contraceptive such as Gedarel ED -28 increases your risk of developing a blood clot compared with not using one. In rare cases a blood clot can block blood vessels and cause serious problems.
Blood clots can develop
- in veins (referred to as a 'venous thrombosis', 'venous thromboembolism' or VTE)
- in the arteries (referred to as an 'arterial thrombosis', 'arterial thromboembolism' or ATE).
Recovery from blood clots is not always complete. Rarely, there may be serious lasting effects or, very rarely, they may be fatal.
It is important to remember that the overall risk of a harmful blood clot due to Gedarel ED -28 is small.
HOW TO RECOGNISE A BLOOD CLOT
Seek urgent medical attention if you notice any of the following signs or symptoms.
|
Are you experiencing any of these signs? |
What are you possibly suffering from? |
|
- swelling of one leg or along a vein in the leg or foot especially when accompanied by: - pain or tenderness in the leg which may be felt only when standing or walking - increased warmth in the affected leg - change in colour of the skin on the leg e.g. turning pale, red or blue |
Deep vein thrombosis |
|
- sudden unexplained breathlessness or rapid breathing; - sudden cough without an obvious cause, which may bring up blood; - sharp chest pain which may increase with deep breathing; - severe light headedness or dizziness; - rapid or irregular heartbeat - severe pain in your stomach; |
Pulmonary embolism |
|
If you are unsure, talk to a doctor as some of these symptoms such as coughing or being short of breath may be mistaken for a milder condition such as a respiratory tract infection (e.g. a 'common cold'). | |
|
Symptoms most commonly occur in one eye: - immediate loss of vision or - painless blurring of vision which can progress to loss of vision |
Retinal vein thrombosis (blood clot in the eye) |
|
- chest pain, discomfort, pressure, heaviness; - sensation of squeezing or fullness in the chest, arm or below the breastbone; - fullness, indigestion or choking feeling; - upper body discomfort radiating to the back, jaw, throat, arm and stomach; - sweating, nausea, vomiting or dizziness; - extreme weakness, anxiety, or shortness of breath; - rapid or irregular heartbeats |
Heart attack |
|
- sudden weakness or numbness of the face, arm or leg, especially on one side of the body; - sudden confusion, trouble speaking or understanding; - sudden trouble seeing in one or both eyes; - sudden trouble walking, dizziness, loss of balance or coordination; - sudden, severe or prolonged headache with no known cause; - loss of consciousness or fainting with or without seizure. |
Stroke |
|
Sometimes the symptoms of stroke can be brief with an almost immediate and full recovery, but you should still seek urgent medical attention as you may be at risk of another stroke. | |
|
- swelling and slight blue discolouration of an extremity; - severe pain in your stomach (acute abdomen). |
Blood clots blocking other blood vessels |
BLOOD CLOTS IN A VEIN
What can happen if a blood clot forms in a vein?
- The use of combined hormonal contraceptives has been connected with an increase in the risk of blood clots in the vein (venous thrombosis). However, these side effects are rare. Most frequently, they occur in the first year of use of a combined hormonal contraceptive.
- If a blood clot forms in a vein in the leg or foot it can cause a deep vein thrombosis (DVT).
- If a blood clot travels from the leg and lodges in the lung it can cause a pulmonary embolism.
- Very rarely a clot may form in a vein in another organ such as the eye (retinal vein thrombosis).
When is the risk of developing a blood clot in a vein highest?
The risk of developing a blood clot in a vein is highest during the first year of taking a combined hormonal contraceptive for the first time. The risk may also be higher if you restart taking a combined hormonal contraceptive (the same product or a different product) after a break of 4 weeks or more.
After the first year, the risk gets smaller but is always slightly higher than if you were not using a combined hormonal contraceptive.
When you stop Gedarel ED -28 your risk of a blood clot returns to normal within a few weeks.
What is the risk of developing a blood clot?
The risk depends on your natural risk of VTE and the type of combined hormonal contraceptive you are taking.
The overall risk of a blood clot in the leg or lung (DVT or PE) with Gedarel ED -28 is small.
- Out of 10,000 women who are not using any combined hormonal contraceptive and are not pregnant, about 2 will develop a blood clot in a year.
- Out of 10,000 women who are using a combined hormonal contraceptive that contains levonorgestrel, norethisterone, or norgestimate about 5-7 will develop a blood clot in a year.
- Out of 10,000 women who are using a combined hormonal contraceptive that contains desogestrel such as Gedarel ED -28 between about 9 and 12 women will develop a blood clot in a year.
- The risk of having a blood clot will vary according to your personal medical history (see "Factors that increase your risk of a blood clot” below).
|
Risk of developing a blood clot in a year | |
|
Women who are not using a combined hormonal pill/patch/ring and are not pregnant |
About 2 out of 10,000 women |
|
Women using a combined hormonal contraceptive pill containing levonorgestrel, norethisterone or norgestimate |
About 5-7 out of 10,000 women |
|
Women using Gedarel ED -28 |
About 9-12 out of 10,000 women |
Factors that increase your risk of a blood clot in a vein
The risk of a blood clot with Gedarel ED -28 is small but some conditions will increase the risk. Your risk is higher:
- if you are very overweight (body mass index or BMI over 30kg/m2);
- if one of your immediate family has had a blood clot in the leg, lung or other organ at a young age (e.g. below the age of about 50). In this case you could have a hereditary blood clotting disorder.
- if you need to have an operation, or if you are off your feet for a long time because of an injury or illness, or you have your leg in a cast. The use of Gedarel ED -28 may need to be stopped several weeks before surgery or while you are less mobile. If you need to stop Gedarel ED -28 ask your doctor when you can start using it again.
- as you get older (particularly above about 35 years);
- if you gave birth less than a few weeks ago.
The risk of developing a blood clot increases the more conditions you have.
Air travel (>4 hours) may temporarily increase your risk of a blood clot, particularly if you have some of the other factors listed.
It is important to tell your doctor if any of these conditions apply to you, even if you are unsure. Your doctor may decide that Gedarel ED -28 needs to be stopped.
If any of the above conditions change while you are using Gedarel ED -28, for example a close family member experiences a thrombosis for no known reason; or you gain a lot of weight, tell your doctor.
BLOOD CLOTS IN AN ARTERY
What can happen if a blood clot forms in an artery?
Like a blood clot in a vein, a clot in an artery can cause serious problems. For example, it can cause a heart attack or a stroke.
Factors that increase your risk of a blood clot in an artery
It is important to note that the risk of a heart attack or stroke from using Gedarel ED -28 is very small but can increase:
- with increasing age (beyond about 35 years);
- if you smoke. When using a combined hormonal contraceptive like Gedarel ED -28 you are advised to stop smoking. If you are unable to stop smoking and are older than 35 your doctor may advise you to use a different type of contraceptive.
- if you are overweight;
- if you have high blood pressure;
- if a member of your immediate family has had a heart attack or stroke at a young age (less than about 50). In this case you could also have a higher risk of having a heart attack or stroke.
- if you, or someone in your immediate family, have a high level of fat in the blood (cholesterol or triglycerides);
- if you get migraines, especially migraines with aura;
- if you have a problem with your heart (valve disorder, disturbance of the rhythm called atrial fibrillation);
- if you have diabetes.
If you have more than one of these conditions or if any of them are particularly severe the risk of developing a blood clot may be increased even more.
If any of the above conditions change while you are using Gedarel ED -28, for example you start smoking, a close family member experiences a thrombosis for no known reason; or you gain a lot of weight, tell your doctor.
The pill and cancer
An increased risk of cervical carcinoma in long-term users of oral contraceptives has been reported in some epidemiological studies, but there is controversy about the extent to which this finding is attributable to the confounding effects of sexual behaviour and other factors such as human papilloma virus (HPV).
Breast cancer has been observed slightly more often in women using combined pills, but it is not known whether this is caused by the treatment. For example it may be that more tumours are detected in women on combined pills because they are examined by their doctor more often. The occurrence of breast tumours becomes gradually less after stopping the combination hormonal contraceptives. It is important to regularly check your breasts and you should contact your doctor if you feel any lump.
In rare cases, benign liver tumours, and in even fewer cases malignant liver tumours have been reported in pill users. Contact your doctor if you have unusual severe abdominal pain.
Children and adolescents
The safety and efficacy of desogestrel in adolescents below 18 years has not yet been established. No data are available.
Other medicines and Gedarel ED -28
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Also tell any other doctor or dentist who prescribes another medicine (or the dispensing pharmacist) that you use Gedarel ED -28. They can tell you if you need to take additional contraceptive precautions (for example condoms) and if so, for how long.
Some medicines can have an influence on the blood levels of Gedarel ED -28 and can make it less effective in preventing pregnancy, or can cause unexpected bleeding. These include medicines used for the treatment of
- epilepsy (e.g. barbiturates, carbamazepine, phenytoin, primidone, felbamate, oxcarbazepine, topiramate);
- tuberculosis (e.g. rifampicin);
- HIV and hepatitis C virus infections (HCV) (so-called protease inhibitors and nonnucleoside reverse transcriptase inhibitors such as ritonavir, nevirapin, efavirenz);
- fungal infections (e.g. griseofulvin);
- increase of blood pressure in the lung vasculature (bosentan);
- the symptomatic treatment of arthrosis (etoricoxib);
- the herbal remedy St. John's wort. If you want to use herbal products containing St. John's wort while you are already using Gedarel ED -28 you should consult your doctor first.
Gedarel ED -28 may also influence the efficacy of other medicines, e.g.
- ciclosporin (medicine used for the treatment of suppression of tissue rejection following transplant surgery),
- lamotrigine (anti-epileptic medicine; this could lead to an increased frequency of seizures),
- tizanidine (medicine used for the treatment of muscle spasticity),
- levothyroxine (medicine used for the treatment of hormone deficiency).
Ask your doctor or pharmacist for advice before taking any medicine.
Before you have any laboratory tests
Tell your doctor or the laboratory staff that you are taking the pill, because oral contraceptives can affect the results of some tests.
Pregnancy and breast-feeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
You must not use Gedarel ED -28 when you are pregnant. If you become pregnant or you think you might be pregnant, stop taking Gedarel ED -28 and talk to your doctor immediately.
Gedarel ED -28 should not be taken during breast-feeding. If you are breast feeding and want to take the pill, you should discuss this with your doctor.
Driving and using machines
You can drive or operate machinery while taking Gedarel ED -28.
Gedarel ED -28 contains lactose and sunset yellow
If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicine.
The ingredient sunset yellow may cause allergic reactions.
3. How to take Gedarel ED -28
Always take this medicine exactly as your doctor has told you. Check with your doctor if you are not sure. If before you started Gedarel ED -28, you took another contraceptive pill, you know that most contraceptive pills contain 21 tablets. With these contraceptive pills, you take one tablet for 21 days and then have a gap (tablet-free) week of 7 days. The pattern when using Gedarel ED -28 is different. After the 21 slightly yellow, active tablets, you must continue right away with the 7 green placebo tablets; there is therefore no gap week but a "placebo” week.
Preparation of the strip
You must know on which day of the week you will take the first tablet.
To help you keep track, there are 7 weekdays sticker strips marked with the 7 days of the week. Choose the weekdays sticker strip that starts with the day you begin taking the tablets. For example, if you start on a Wednesday, use the weekdays sticker strip that starts with "Wed”.
Fit the symbol on the strip to the same symbol on the blister card and place the strip into the area bordered with black line. Each day will line up with a row of pills. There is now a day shown above every tablet and you can see whether you have taken a pill on a particular day. Follow the direction of the arrow on the wallet until all 28 tablets have been taken.
Following the direction of the arrow printed on the strip you should take one tablet each day for 28 days until the strip is empty.
During the 7 days that you are taking the green placebo tablets (the placebo week), bleeding should begin (so-called withdrawal bleeding). This usually starts on the 2nd or 3rd day after the last slightly yellow active tablet of Gedarel ED -28. Once you have taken the last green tablet, you should start with the following strip, whether your bleeding has stopped or not. This means that you should start the following strip on the same day of the week, and that the withdrawal bleed should occur on the same days each month.
If you use Gedarel ED -28 in this manner, you are protected against pregnancy also during the 7 days that you are taking a placebo tablet.
You should try to take your tablet at about the same time each day. You may find it easiest to take it either last thing at night or first thing in the morning.
Swallow each tablet whole, with water if necessary.
Starting the first pack
If you have not used a contraceptive with hormones in the previous month
Take the first tablet on the first day of your period. This is the first day of your cycle -the day when bleeding starts. Take the first tablet and follow the direction of the arrow and continue taking one tablet each day until the strip is empty.
If you start on day 2-5 of your period, you should use another method of contraception as well, such as the condom, for the first seven tablet-taking days, but this is only for the first pack.
Changing to Gedarel ED -28 from another combined hormonal contraceptive, or combined contraceptive vaginal ring or patch
Start taking Gedarel ED -28 on the day after the tablet-free period of your previous pill finishes (or after the last inactive tablet of your previous pill). If a vaginal ring or a transdermal patch has been used, you should start using Gedarel ED -28 preferably on the day of removal of the ring/patch, but at the latest when the next application/ insertion would have been due.
Changing to Gedarel ED -28 from a progestogen-product (progestogen-only-pills, injection, implant or progestogen releasing IUD)
You may switch any day from the progestogen-only tablet (from an implant or the IUD on the day of its removal, from an injectable when the next injection would be due) but in all of these cases you must use extra protective measures (for example, a condom) for the first 7 days of tablet-taking.
After a miscarriage or abortion
Follow the advice of your doctor.
After having a baby
After having a baby, you can start Gedarel ED -28 between 21 and 28 days later.
If you start later than day 28, you must use a so-called barrier method (for example, a condom) during the first seven days of Gedarel ED -28 use. If, after having a baby, you have had intercourse before starting Gedarel ED -28 (again), you must first be sure that you are not pregnant or you must wait until the next menstrual bleed.
Let your doctor advise you, in case you are not sure when to start.
If you are breast-feeding and want to start Gedarel ED -28 (again) after having a baby.
Read the section on "Pregnancy and breast-feeding”.
If you take more Gedarel ED -28 than you should
There are no reports of serious harmful results of taking too many Gedarel ED -28 tablets. If you take several tablets at once then you may have symptoms of nausea or vomiting. Young girls may have bleeding from the vagina. If you have taken too many Gedarel ED -28 tablets, or you discover that a child has taken some, ask your doctor or pharmacist for advice.
If you forget to take Gedarel ED -28
The green tablets of the strip are placebo tablets. If you forget one these tablets, this has no effect on the reliability of Gedarel ED -28. You should throw away the forgotten placebo tablet so that you do not make the placebo week longer. This might have a negative effect on the reliability of Gedarel ED -28.
If you miss a slightly yellow tablet, you must follow the following advice:
- If you are less than 12 hours late taking a tablet, the protection from pregnancy is not reduced. Take the tablet as soon as you remember and then take the following tablets again at the usual time.
- If you are more than 12 hours late taking a tablet, the protection from pregnancy may be reduced. The greater the number of tablets that you have forgotten, the greater is the risk that the protection from pregnancy is reduced.
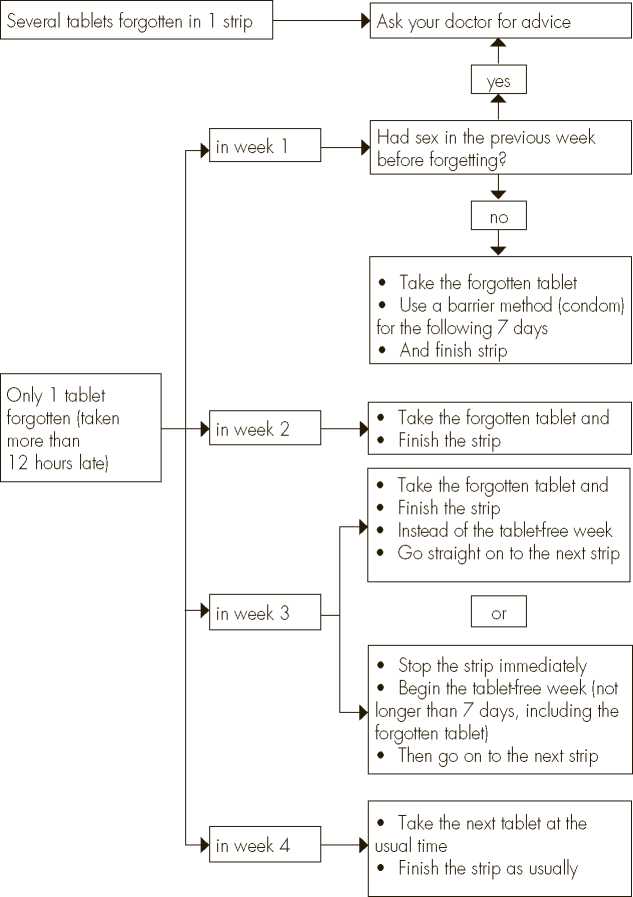
The risk of incomplete protection against pregnancy is greatest if you forget a tablet at the beginning or the end of the strip. Therefore, you should adhere to the following rules (see also the diagram below):
More than one tablet forgotten in this strip
Contact your doctor.
One tablet forgotten in week 1
Take the forgotten tablet as soon as you remember, even if that means that you have to take two tablets at the same time. Take the tablets again at the usual time and use extra precautions for the next 7 days, for example, a condom. If you have had intercourse in the week before the oversight or you have forgotten to start a new strip after the placebo tablets period, you must realize that there is a risk of pregnancy. In that case, contact your doctor.
One tablet forgotten in week 2
Take the forgotten tablet as soon as you remember, even if that means that you have to take two tablets at the same time. Take the tablets again at the usual time. Provided that the tablets have been taken correctly for the 7 days preceding the forgotten tablet, the protection from pregnancy is not reduced, and you do not need to take extra precautions.
One tablet forgotten in week 3
You can choose between two possibilities:
1. Take the forgotten tablet as soon as you remember, even if that means that you have to take two tablets at the same time. Take the tablets again at the usual time. Instead of taking the green tablets (placebo tablets), go straight on to the next strip. Most likely, you will have a period (withdrawal bleed) at the end of the second strip -during taking the green tablets- but you may also have spotting or breakthrough bleeding during the second strip.
2. You can also stop taking the slightly yellow tablets and go directly on to the
7 green placebo tablets (record the day on which you forgot your tablet). If you
want to start a new strip on your fixed start day, make the placebo period less than 7 days.
If you follow either of these two recommendations, you will remain protected against pregnancy.
One tablet forgotten in week 4
The contraceptive effect is not reduced and you should take the subsequent tablets at the usual time.
If you have forgotten any of the tablets in a strip, and you do not have bleeding in the normal placebo week, this may mean that you are pregnant. You must contact your doctor before you go on to the next strip.
What to do in case of vomiting or severe diarrhoea
If you vomit within 3-4 hours of taking a tablet or you have severe diarrhoea, there is a risk that the active substances in the tablet are not fully absorbed into your body. The situation is similar to if you forget a tablet. After the vomiting or diarrhoea has stopped, you must take another tablet from a reserve strip as soon as possible. This should be done within 12 hours of when you normally take your tablet. If this is not possible and more than 12 hours have passed, you should follow the advice given under "If you forget to take Gedarel ED -28".
Bleeding between periods
During the first few months that you are taking Gedarel ED -28, you may have unexpected bleeding (bleeding outside the placebo period). If this bleeding lasts longer than a few months, or if it begins after some months, your doctor must investigate the cause.
What you must do if no bleeding occurs in the placebo-tablet week
If you have taken all the tablets correctly, have not had vomiting or severe diarrhoea and you have not taken any other medicines, it is highly unlikely that you are pregnant.
If the expected bleeding does not happen twice in succession, you may be pregnant. Contact your doctor immediately. Do not start the next strip until you are sure that you are not pregnant.
Ask your doctor for advice.
Delay of menstrual period: what you must know
Even if not recommended, delay of your menstrual period (withdrawal bleed) is possible by going straight on to a new strip of Gedarel ED -28 after the last slightly yellow active tablet instead of taking the green placebo tablets. You may experience spotting (drops or flecks of blood) or breakthrough bleeding while using this second strip. After the usual placebo-tablet period of 7 days, continue with the following strip. You might ask your doctor for advice before deciding to delay your menstrual period.
Change of the first day of your menstrual period: what you must know
If you take the tablets according to the instructions, then your menstrual period/ withdrawal bleed will begin whilst taking the placebo-tablets. If you have to change this day, you do this by making the placebo-tablet period shorter (but never longer!). For example, if your placebo-tablet period begins on a Friday, and you want to change this to a Tuesday (3 days earlier) you must start a new strip 3 days earlier than usual. If you make the placebo-tablet period very short (for example, 3 days or less) then it may be that you do not have any bleeding during this placebo-tablet period. You may then experience spotting (droplets or flecks or blood) or breakthrough bleeding.
If you are not sure how to proceed, contact your doctor for advice.
If you stop taking Gedarel ED -28
You can stop taking Gedarel ED -28 whenever you want. If you do not want to become pregnant, ask your doctor for advice about other reliable methods of birth control.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. If you get any side effect, particularly if severe and persistent, or have any change to your health that you think may be due to Gedarel ED -28, please talk to your doctor.
An increased risk of blood clots in your veins (venous thromboembolism (VTE)) or blood clots in your arteries (arterial thromboembolism (ATE)) is present for all women taking combined hormonal contraceptives. For more detailed information on the different risks from taking combined hormonal contraceptives please see section 2 "What you need to know before you take Gedarel ED -28".
Very common (may affect more than 1 in 10 people): irregular bleeding.
Common (may affect up to 1 in 10 people): depression, mood altered, nervousness, headache, dizziness, nausea, abdominal pain, acne, tender breasts, breast pain, absence of menstruation, painful menstruation, pre-menstrual syndrome (physical and emotional problems before the start of menstruation), weight gain.
Uncommon (may affect up to 1 in 100 people): fluid retention, decreased sexual desire, migraine, impaired hearing (otosclerosis), high blood pressure, diarrhoea, vomiting, rash, nettle-rash (urticaria), breast enlargement.
Rare (may affect up to 1 in 1,000 people): hypersensitivity, increased sexual desire, eye irritation due to contact lens, skin disorders (erythema nodosum - a skin disease associated with joint pain, fever, hypersensitivity, or infection, and characterized by small, painful, pink to blue nodules under the skin and on the shins that tend to recur, erythema multiforme - a skin disease characterized by solid raised spots on the skin or fluid-filled blisters lesions and reddening or discoloration of the skin often in concentric zones about the lesions), chloasma (discolouration of the skin, so called "pregnancy patches"), vaginal discharge, breast discharge and weight loss.
- Harmful blood clots in a vein or artery for example:
- in a leg or foot (i.e. DVT)
- in a lung (i.e. PE)
- heart attack
- stroke
- mini-stroke or temporary stroke-like symptoms, known as a transient ischaemic attack (TIA)
- blood clots in the liver, stomach/intestine, kidneys or eye.
The chance of having a blood clot may be higher if you have any other conditions that increase this risk (see section 2 for more information on the conditions that increase risk for blood clots and the symptoms of a blood clot).
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via Yellow Card Scheme. Website: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Gedarel ED -28
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after EXP. The expiry date refers to the last day of that month.
Store below 30°C, in the original packaging in order to protect from light.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use. These measures will help protect the environment.
6. Contents of the pack and other information What Gedarel ED -28 contains
The active substances are ethinylestradiol and desogestrel in each slightly yellow tablet.
One slightly yellow film-coated tablet contains 20 micrograms ethinylestradiol and 150 micrograms desogestrel.
The green placebo (inactive) film-coated tablet does not contain active substances.
The other ingredients are:
Active film-coated tablets:
Tablet core: potato starch; stearic acid; all-rac-alpha-tocopherol; lactose monohydrate; magnesium stearate; silica colloidal anhydrous; povidone K 30, quinoline yellow (E104).
Tablet coating: hypromellose; macrogol 6000; propylene glycol.
Placebo film-coated tablets:
Tablet core: cellulose, microcrystalline; lactose anhydrous, maize starch, pregelatinised, magnesium stearate, silica, colloidal anhydrous.
Tablet coating: polyvinyl alcohol, titanium dioxide (E171), macrogol 3350, talc, Indigo Carmine (E132), Quinoline Yellow (E104), iron oxide, black (E172), Sunset Yellow (E110).
What Gedarel ED -28 looks like and contents of the pack
The active film-coated tablet: slightly yellow, round shaped, biconvex film-coated tablets of about 6 mm diameter, with P9 sign on one side and RG sign on other side.
The placebo film-coated tablet is green, round, biconvex film-coated tablet, diameter about 6 mm, without engraving.
Gedarel ED 20 micrograms/150 micrograms -28 film-coated tablets are packed into the PVC/PVDC//Aluminium blisters. The blisters are packed into cardboard box with a patient information leaflet, an etui storage bag and weekdays sticker(s) enclosed in each box.
Each box contains 1, 3, 6 or 13 calendar pack(s) of 21 active film-coated tablets+7 placebo film-coated tablet.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
Gedeon Richter Plc.
H-1103 Budapest Gyomroi ut 19-21.
Hungary
This medicinal product is authorised in the Member States of the EEA under the following names:
Denmark: Myrzi 28 Belgium: DESO 20 CONTINU Luxembourg: DESO 20 CONTINU United Kingdom: Gedarel ED-28
This leaflet was last revised in May 2016.
K-23308-1.3
© GEDEON RICHTER
K> J2
O 0
3
n'
_Q
O
m l*-1
> m
TO
m l*-1
Q M
CD O
oT 04