Genotropin Miniquick 0.2Mg Powder And Solvent For Solution For Injection

PAA056915
81
m
0.2 mg, 0.4 mg, 0.6 mg, 0.8 mg, 1.0 mg, 1.2 mg, 1.4 mg, 1.6 mg, 1.8 mg, 2.0 mg
PACKAGE LEAFLET: INFORMATION FOR THE USER
Cenotropin
powder and solvent for solution for injection
somatropin
Read all of this leaflet carefully before you start using this medicine.
- Keep this leaflet. You may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What Genotropin MiniQuick is and what it is used for
2. Before you use Genotropin MiniQuick
3. Howto use Genotropin MiniQuick
4. Possible side effects
5 How to store Genotropin MiniQuick 6. Further information
1. What Genotropin MiniQuick is and what it is used for
Genotropin MiniQuick is a recombinant human growth hormone (also called somatropin). It has the same structure as natural human growth hormone which is needed for bones and muscles to grow. It also helps your fat and muscle tissues to develop in the right amounts. It is recombinant meaning it is not made from human or animal tissue.
In children, Genotropin MiniQuick is used to treat growth disturbances:
• If you are not growing properly and you do not have enough of your own growth hormone.
• If you have Turner syndrome. Turner syndrome is a chromosomal error in girls that can affect growth - your doctor will have told you if you have this.
• If you have chronic renal (kidney) insufficiency. As kidneys lose their ability to function normally, this can affect growth.
• If you have Prader-Willi syndrome (a chromosomal disorder). Growth hormone will help you grow taller if you are still growing, and will also improve your body composition. Your excessive fat will decrease and your reduced muscle mass will improve.
• If you were small or too light at birth. Growth hormone can help you grow taller if you have not been able to catch up or maintain normal growth by four years of age or later.
In adults, Genotropin MiniQuick is used to treat persons with pronounced growth hormone deficiency. This can start during adult life, or it can continue from childhood.
If you have been treated with Genotropin MiniQuick for growth hormone deficiency during childhood, your growth hormone status will be retested after completion of growth. If severe growth hormone deficiency is confirmed, your doctor will propose continuation of Genotropin MiniQuick treatment. You should only be given this medicine by a doctor who has experience with growth hormone treatment and who has confirmed your diagnosis.
2. Before You Use Genotropin Miniquick
Do not use Genotropin MiniQuick and tell your doctor if
• You are allergic (hypersensitive) to somatropin or any of the other ingredients of Genotropin MiniQuick.
• You have an active tumour (cancer). Tumours must be inactive and you must have finished your anti-tumour treatment before you start your treatment with Genotropin MiniQuick.
• You are seriously ill (for example, complications following open heart surgery, abdominal surgery, acute respiratory failure, accidental trauma or similar conditions). If you are about to have, or have had, a major operation, or go into hospital for any reason, tell your doctor and remind the other doctors you are seeing that you use growth hormone.
• Genotropin MiniQuick has been prescribed to stimulate growth but you have already stopped growing (closed epiphyses).
Take special care with Genotropin MiniQuick and tell your doctor if any of the following
statements apply to you
• If you are at risk of developing diabetes, your doctor will need to monitor your blood sugar level during treatment with Genotropin MiniQuick.
• If you have diabetes, you should closely monitor your blood sugar level during treatment with Genotropin MiniQuick and discuss the results with your doctor to determine whether you need to change the dose of your medicines to treat diabetes.
• After starting Genotropin treatment some patients may need to start thyroid hormone replacement.
• If you are receiving treatment with thyroid hormones it may be necessary to adjust your thyroid hormone dose.
• If you are taking growth hormone to stimulate growth and walk with a limp or if you start to limp during your growth hormone treatment due to pain in your hip, you should inform your doctor.
• If you develop raised intracranial pressure (with symptoms such as strong headache, visual disturbances or vomiting) you should inform your doctor about it.
• If you are receiving Genotropin MiniQuick for growth hormone deficiency following a previous tumour (cancer), you should be examined regularly for recurrence of the tumour or any other cancer.
• If you experience worsening abdominal pain you should inform your doctor.
• Experience in patients above 80 years of age is limited. Elderly persons may be more sensitive to the action of Genotropin MiniQuick, and therefore may be more prone to develop side effects.
Children with chronic renal (kidney) insufficiency:
• Your doctor should examine your kidney function and your growth rate before starting Genotropin MiniQuick. Medical treatment for your kidney condition should be continued. Genotropin MiniQuick treatment should be stopped at kidney transplantation.
Children with Prader-Willi syndrome:
• Your doctor will give you diet restrictions to follow to control your weight.
• Your doctor will assess you for signs of upper airway obstruction, sleep apnoea (where your breathing is interrupted during sleep), or respiratory infection before you start treatment with Genotropin MiniQuick.
• During treatment, if you show signs of upper airway obstruction (including starting to snore or worsening of snoring), your doctor will need to examine you and may interrupt your treatment with Genotropin MiniQuick.
• During treatment, your doctor will check you for signs of scoliosis, a type of spinal deformity.
• During treatment, if you develop a lung infection, tell your doctor so that he can treat the infection.
Children born small or too light at birth:
• If you were small or too light at birth and are aged between 9 and 12 years, ask your doctor for specific advice relating to puberty and treatment with this product.
• Your doctor will check your blood sugar and insulin levels before the start of treatment and every year during treatment.
• Treatment should be continued until you have stopped growing.
Using other medicines
Please tell your doctor or pharmacist if you are using or have recently used any other medicines, including medicines obtained without a prescription.
You should tell your doctor if you are using:
• medicines to treat diabetes,
• thyroid hormones,
• synthetic adrenal hormones (corticosteroids),
• sex hormones (for example oestrogens),
• ciclosporin (a medicine that weakens the immune system after transplantation),
• medicines to control epilepsy (anticonvulsants).
Your doctor may need to adjust the dose of these medicines or the dose of Genotropin MiniQuick. Pregnancy and breast-feeding
You should not use Genotropin if you are pregnant, think you may be pregnant or are trying to become pregnant.
Ask your doctor for advice before using this medicine while breast-feeding.
Ask your doctor or pharmacist for advice before taking any medicine.
Important information about some of the ingredients of Genotropin MiniQuick
This medicine contains less than 1 mmol sodium (23 mg) per dose, i.e. essentially ‘sodium-free’.
3. How To Use Genotropin Miniquick
Recommended dosage
The dose depends on your size, the condition for which you are being treated and how well growth hormone works for you. Everyone is different. Your doctor will advise you about your individualised dose of Genotropin MiniQuick in milligrams (mg) from either your body weight in kilograms (kg) or your body surface area calculated from your height and weight in square metres (m£), as well as your treatment schedule. Do not change the dosage and treatment schedule without consulting your doctor.
Children with growth hormone deficiency:
0.025-0.035 mg/kg bodyweight per day or 0.7-1.0 mg/m! body surface area per day. Higher doses can be used. When growth hormone deficiency continues into adolescence, Genotropin MiniQuick should be continued until completion of physical development.
Children with Turner syndrome:
0.045-0.050 mg/kg bodyweight per day or 1.4 mg/m! body surface area per day.
Processstructur Black I
Children with chronic renal (kidney) insufficiency:
0.045-0.050 mg/kg bodyweight per day or 1.4 mg/nf body surface area per day. Higher doses may be necessary if the rate of growth is too low. Dosage adjustment may be necessary after 6 months of treatment.
Children with Prader-Willi syndrome:
0.035 mg/kg bodyweight per day or 1.0 mg/nf body surface area per day. The daily dosage should not exceed 2.7 mg. Treatment should not be used in children who have almost stopped growing after puberty.
Children born smaller or lighter than expected and with growth disturbance:
0.035 mg/kg bodyweight per day or 1.0 mg/nf body surface area per day. It is important to continue treatment until final height is reached. Treatment should be discontinued after the first year if you are not responding or if you have reached your final height and stopped growing.
Adults with growth hormone deficiency:
If you continue Genotropin MiniQuick after treatment during childhood you should start with 0.2-0.5 mg per day. This dosage should be gradually increased or decreased according to blood test results as well as clinical response and side effects.
If your growth hormone deficiency starts during adult life you should start with 0.15-0.3 mg per day. This dosage should be gradually increased according to blood test results as well as clinical response and side effects. The daily maintenance dose seldom exceeds 1.0 mg per day. Women may reguire higher doses than men. Dosage should be monitored every 6 months. Persons above 60 years should start with a dose of 0.1 -0.2 mg per day which should be slowly increased according to individual reguirements. The minimum effective dose should be used. The maintenance dose seldom exceeds 0.5 mg per day. Follow the instructions given to you by your doctor.
Injecting Genotropin MiniQuick
Genotropin MiniQuick is intended for subcutaneous use. This means that it is injected through a short injection needle into the fatty tissue just under your skin. Your doctor should have already shown you how to use Genotropin MiniQuick. Always inject Genotropin MiniQuick exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
Please see the “Instructions for Use” at the end of the package leaflet for advice on how to use your Genotropin MiniQuick. If you cannot remember what to do, do not try to do your injection. Ask your doctor to show you again.
You can take your growth hormone out of the refrigerator half an hour before your injection. This lets it warm up slightly and can make your injections more comfortable.
Remember to wash your hands and clean your skin first.
Inject your growth hormone at about the same time every day. Bedtime is a good time because it is easy to remember. It is also natural to have a higher level of growth hormone at night.
Most people do their injections into their thigh or their bottom. Do your injection in the place you have been shown by your doctor. Fatty tissue of the skin can shrink at the site of injection. To avoid it, use a slightly different place for your injection each time. This gives your skin and the area under your skin time to recover from one injection before it gets another one in the same place.
If you use more Genotropin MiniQuick than you should
If you inject much more than you should, contact your doctor or pharmacist as soon as possible. Your blood sugar level could fall too low and later rise too high. You might feel shaky, sweaty, sleepy or “not yourself”, and you might faint.
If you forget to use Genotropin MiniQuick
Do not take a double dose to make up for a forgotten dose.
It is best to take your growth hormone regularly. If you forget to take a dose, have your next injection at the usual time the next day. Keep a note of any missed injections and tell your doctor at your next check-up.
If you stop using Genotropin MiniQuick
Ask for advice from your doctor before you stop using Genotropin MiniQuick.
If you have any further guestions on the use of this product, ask your doctor or pharmacist.
4. Possible Side Effects
Like all medicines, Genotropin MiniQuick can cause side effects, although not everybody gets them. The very common and common side effects in adults may start within the first months of treatment and may either stop spontaneously or if your dose is reduced.
Very common side effects (likely to occur in more than 1 in 10 patients) include:
In adults
• Joint pain
• Water retention (which shows as puffy fingers or swollen ankles).
Common side effects (likely to occur in fewer than 1 in 10 patients) include:
In children:
• Temporary reddening, itchiness or pain at the injection site.
• Joint pain In adults:
• Numbness/tingling,
• Stiffness in the arms and legs, muscle pain,
• Pain or burning sensation in the hands or underarms (known as Carpal Tunnel Syndrome) Uncommon side effects (likely to occur in fewer than 1 in 100 patients) include:
In children:
• Water retention (which shows as puffy fingers or swollen ankles, for a short time at the start of treatment). Rare side effects (likely to occur in fewer than 1 in 1,000 patients) include:
In children:
• Numbness/tingling,
• Leukaemia (This has been reported in a small number of growth hormone deficiency patients, some of whom have been treated with somatropin. However, there is no evidence that leukaemia incidence is increased in growth hormone recipients without predisposing factors).
• Increased intracranial pressure (which causes symptoms such as strong headache, visual disturbances or vomiting).
• Muscle pain
Not known: frequency cannot be estimated from the available data
• Type 2 diabetes.
• A decrease in the levels of the hormone Cortisol in your blood.
In children:
• Stiffness in the arms and legs

In adults:
• Increased intracranial pressure (which causes symptoms such as strong headache, visual disturbances or vomiting).
• Reddening, itchiness or pain at the injection site
Formation of antibodies to the injected growth hormone but these do not seem to stop the growth hormone from working.
The skin around the injection area can get uneven or lumpy, but this should not happen if you inject in a different place each time.
There have been rare cases of sudden death in patients with Prader-Willi syndrome. However, no link has been made between these cases and treatment with Genotropin MiniQuick.
Slipped capital femoral epiphysis and Legg-Calve-Perthes disease may be considered by your doctor if discomfort or pain in the hip or knee is experienced whilst being treated with Genotropin.
Other possible side effects related to your treatment with growth hormone may include the following. You (or your child) may experience a high blood sugar or reduced levels of thyroid hormone. This can be tested by your doctor and if necessary your doctor will prescribe the adequate treatment. Rarely, an inflammation of the pancreas has been reported in patients treated with growth hormone.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly (see details below). By reporting side effects you can help provide more information on the safety of this medicine.
United Kingdom
Yellow Card Scheme website: www.mhra.gov.uk/yellowcard Ireland
HPRA Pharmacovigilance,
Earlsfort Terrace,
IRL-Dublin 2;
Tel:+353 1 676 4971;
Fax: +353 1 676 2517.
Website: www.hpra.ie; e-mail: medsafety@hpra.ie.
5. How To Store Genotropin Miniquick
Keep out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton as MM/YYYY. The expiry date refers to the last day of that month.
Before reconstitution:
Store in a refrigerator (2°C - 8°C). Do not freeze. Keep the syringe in the outer carton in order to protect from light.

Ireland PA Holder:
Pfizer Healthcare Ireland,
9 Riverwalk, National Digital Park Citywest Business Campus Dublin 24, Ireland
Before opening, the product may be taken out of the refrigerator, without being replaced, for a maximum period of 6 months at a temperature not above 25°C. The date when the medicine is taken out and the new expiry date should be written on the outer packaging. This new expiry date should never exceed the one initially mentioned on the outer carton. If the medicine has not been used before the new expiry date, it should be disposed of.
After reconstitution:
Use immediately or store in a refrigerator (2°C - 8°C) for up to 24 hours. Do not freeze. Keep the syringe in the outer carton in order to protect from light.
Do not use this medicine if you notice particles or if the solution is not clear.
Never throw away needles or empty syringes with your ordinary rubbish. When you have finished with a needle, you must discard it carefully so that no-one will be able to use it or prick themselves on it. You can get a special ‘sharps’ bin from your hospital or growth clinic.
Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to throw away medicines you no longer use.
These measures will help protect the environment.
6. Further Information
What Genotropin MiniQuick contains
• The active substance is somatropin*.
• One cartridge contains 0.2 mg, 0.4 mg, 0.6 mg, 0.8 mg, 1.0 mg, 1.2 mg, 1.4 mg, 1.6 mg,
1.8 mg, or 2.0 mg per 0.25 ml of somatropin* after reconstitution, corresponding to a concentration of 0.8 mg, 1.6 mg, 2.4 mg, 3.2 mg, 4 mg, 4.8 mg, 5.6 mg, 6.4 mg, 7.2 mg and 8 mg per ml.
• The other ingredients in the powder are: glycine (E640), mannitol (E421), sodium dihydrogen phosphate anhydrous (E339), and disodium phosphate anhydrous (E339).
• The ingredients in the solvent are: water for injections, and mannitol (E421).
• produced in Escherichia coli cells by recombinant DNA technology.
What Genotropin MiniQuick looks like and contents of the pack
Powder and solvent for solution for injection, in a two-chamber cartridge containing the powder in
one section and the solvent in the other (0.2 mg/0.25 ml, 0.4 mg/0.25 ml, 0.6 mg/0.25 ml,
0.8 mg/0.25 ml, 1.0 mg/0.25 ml, 1.2 mg/0.25 ml, 1.4 mg/0.25 ml, 1.6 mg/0.25 ml,
1.8 mg/0.25 ml or 2.0 mg/0.25 ml). The cartridge is contained in a syringe. Pack size of 4 or 7 or
28 syringes.
Not all strengths and pack sizes may be marketed.
The powder is white and the solvent is clear.
Marketing Authorisation Holder UK PL Holder:
Pfizer Limited,
Ramsgate Road,
Sandwich, Kent,
CT13 9NJ, United Kingdom
Manufacturer
Pfizer Manufacturing Belgium NV Rijksweg 12 2870 Puurs Belgium
This medicinal product is authorised in the Member States of the EEA under the following names: Genotropin MiniQuick: Austria, Denmark, Germany, Greece, Ireland, Italy, Portugal, Sweden, United Kingdom
Genotonorm MiniQuick: Belgium, France, Luxembourg, Spain This leaflet was last approved in:
UK 09/2014 IE 10/2014 Ref: GN28_2
Genotropin MiniQuick Instructions for Use
Genotropin MiniQuick is a syringe used to mix and administer a single dose of Genotropin (growth hormone).
Each Genotropin MiniQuick comes preloaded with a cartridge with two sections and a needle. If you need additional needles, ask for the same Becton Dickinson Micro-Fine needles provided with the MiniQuick. The injection volume is always 0.25 ml.
The Genotropin MiniQuick is disposable; after you have administered a dose, dispose of it as described in step 6.
The diagram below identifies the different components.
Needle Two-chamber
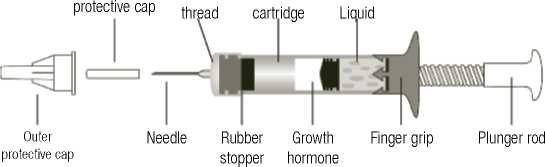
The cartridge of Genotropin MiniQuick contains the growth hormone powder in one section and a liguid in the other. When you turn the plunger rod clockwise, the growth hormone powder and the liguid mix and the powder dissolves.
1 Peel the paper covering from the injection needle. Position the needle sguarely onto the end
of the rubber stopper. Screw the needle onto the Genotropin MiniQuick by turning it clockwise until it no longer can turn.
2 Hold the Genotropin MiniQuick with the needle pointing upwards. Turn the plunger rod
clockwise until it will go no further.
DO NOT shake the solution. Mix it gently.
Shaking the solution could make your growth hormone foam and damage the active substance. Check the solution for clarity, and only use clear solutions that are particle free.
3 Remove the outer and inner protective caps of the needle.
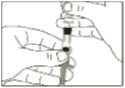
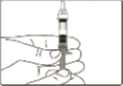
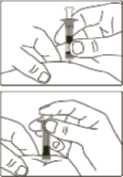
4 Pinch a fold of skin at the injection site firmly and push the needle into the skinfold.
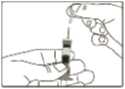
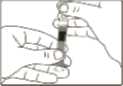
5 Push the plunger rod as far as possible to inject the entire content of the Genotropin MiniQuick. Wait a few seconds before withdrawing the
needle to ensure that all the growth hormone is
6 After injection, do not attempt to replace the protective caps on the needle. Dispose of the syringe with the,
needle, outer and inner protective caps in accordance with standard guidelines or as directed by your doctor or pharmacist.
injected.
QUESTIONS AND ANSWERS
Question
Is it a problem if I see air bubbles in the syringe?
What should I do if there is resistance when I turn the plunger rod (step 2) or when I make the injection (step 5)?
What should I do if the needle is damaged or bent?
Answer
No. There is no need to remove the air from Genotropin MiniQuick. The small amount of air in the syringe has no conseguence on the injection.
The resistance could be because the needle has been inserted at an angle into the rubber stopper.
Carefully replace the outer protective cap (the opague white one) over the needle and unscrew anti-clockwise to remove the needle. Hold the MiniQuick syringe with the needle-end pointing up and reposition the needle sguarely on top of the syringe. Screw the needle into the syringe.
Discard the needle and use a new needle with the MiniQuick.
