Gonapeptyl Depot 3.75Mg Powder And Solvent For Suspension For Injection
2009054567
PACKAGE LEAFLET: INFORMATION FOR THE USER
■
GONAPEPTYL® Depot 3.75mg
Powder and solvent for suspension for injection Triptorelin
Read all of this leaflet carefully before you start treatment with this medicine.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, please ask your doctor or pharmacist.
• If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
In this leaflet:
1. What GONAPEPTYL Depot is and what it is used for
2. Before you are given gOnAPEPTYL Depot
3. How to be given GONAPEPTYL Depot
4. Possible side effects
5. How to store GONAPEPYL Depot
6. Further information
1. WHAT GONAPEPTYL DEPOT IS AND WHAT IT IS USED FOR
GONAPEPTYL Depot contains triptorelin (as triptorelin acetate).Triptorelin belongs to a group of medicines called GnRH analogues. One of its actions is to decrease the production of sex hormones in the body.
It is used:
In Men:
• For the treatment of hormone dependent locally advanced or metastatic prostate cancer.
In Women:
To suppress the levels of ovarian hormones in order to -
• Reduce the size of uterine myomas, (commonly known as fibroids) which are non-cancerous tumours arising from the myometrium (smooth muscle layer) of the uterus.
• Treat endometriosis (the formation of uterine tissue outside the uterus).
In Children:
• For the treatment of central precocious puberty (puberty that occurs prematurely but with the physical and hormonal changes of normal puberty).
2. BEFORE YOU ARE GIVEN GONAPEPTYL DEPOT
You must not be given GONAPEPTYL Depot
• If you are allergic to triptorelin or any of the other ingredients of GONAPEPTYL Depot.
• If you are allergic to gonadotropin-releasing hormone (GnRH) or any other GnRH analogues.
In Women:
• If you are pregnant or are breast feeding.
Take special care with GONAPEPTYL Depot Men and women:
• There have been reports of depression in patients taking Gonapeptyl which may be severe. If you are taking Gonapeptyl and develop depressed mood, inform your doctor.
• As GONAPEPTYL Depot can lead to mood changes.
• As treatment with GONAPEPTYL Depot in rare cases can lead to brain hemorrhage (pituitary apoplexia). Contact your doctor immediately if you experience sudden headache, vomiting or visual disturbances.
• As treatment with GONAPEPTYL Depot can led to thinning of bones which increases risk of bone injury.
• If you are at additional risk of thinning of the bones (osteoporosis) you should tell you doctor before taking GONAPEPTYL Depot. Risk factors include:
- If any of your close family have thinning of the bones.
- If you drink excessive amounts of alcohol, have a poor diet and/or smoke heavily.
- If you are also being treated with certain medicines which may affect the strength of bone.
In Men:
Tell your doctor
• If you have pains in your bones, or difficulty passing urine.
• If you have a secondary spinal or urinary tract tumour.
• If you are castrated.
• if you are diagnosed with diabetes
• If you have a high risk of heart disease, such as diagnosed high blood pressure or heart rhythm problems (arrhythmia).
• If you have any heart or blood vessel conditions, including heart rhythm problems (arrhythmia), or are being treated with medicines for these conditions. The risk of heart rhythm problems may be increased when using GONAPEPTYL.
During treatment:
During the beginning of therapy with GONAPEPTYL Depot you may experiencea worsening in your disease symptoms. Contact your doctor if any of your symptoms of the disease get worse
In Women:
Tell your doctor
• If you are experiencing bleeding mid-cycle during treatment (except for the first month).
During treatmert:
Non-hormorel methods of contraception, such as a condom or a diaphragm, should be used during the first month efter the first injection. It should also be used from 4 weeks after the last injection until the return of your periods (menstruation).
Your periods will stop during treatment. Once treatment has finished, your periods (menstruation) will resume 7-12 weeks after the final injection.
If your periods (menstruation) persists during treatment, please inform your doctor.
In Children:
• Treatment should only be started in girls under 9 years of age and boys under 10 years of age.
During treatment:
In the first month of treatment girls can experience mild to moderate episodes of vaginal bleeding.
After finalising the therapy, development of puberty characteristics will occur. In most girls menses will start on average one year after ending the therapy, which in most cases is regular.
For any possible side effects please see section 4.
Using other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
GONAPEPTYL Depot might interfere with some medicines used to treat heart rhythm problems (e.g. quinidine, procainamide, amiodarone and sotalol) or might increase the risk of heart rhythm problems when used with some other drugs (e.g. methadone (used for pain relief and part of drug addiction detoxification), moxifloxacin (an antibiotic) antipsychotics used for serious mental illnesses).
Pregnancy and lactation
GONAPEPTYL Depot must not be used during pregnancy and lactation (see also the section ‘You must not be given GONAPEPTYL Depot’). If you are possibly pregnant, pregnancy should be ruled out by your doctor before you should use GONAPEPTYL Depot.
Women of childbearing potential should use effective non-hormonal contraception, such as a condom or a diaphragm, during treatment with GONAPEPTYL Depot until menstruation resumes.
Driving and using machines
There are no known effects on the ability to drive or use machinery.
However it cannot be ruled out that the ability to drive or use machinery can be affected during treatment due to some of the side effects (dizziness, sleep disturbances/insomnia and disturbed eye vision. Take extra caution if you experience these side effects.
3. HOW YOU WILL BE GIVEN GONAPEPTYL DEPOT
The powder and solvent are normally mixed and injected by a healthcare professional.
Depending on the condition you are being treated for, the appropriate dose will be administered by intramuscular injection (into a muscle) or subcutaneous injection (just under the skin).
In Men:
• One injection of GONAPEPTYL Depot is normally given every 4 weeks as a long-term therapy.
In Women:
• One injection of GONAPEPTYL Depot is normally given every 4 weeks for up to six months.
• Treatment must be started during the first 5 days of the menstrual cycle.
In Children:
• At the beginning of treatment one injection should be injected on days 0, 14 and 28.
• The dose is adjusted according to body weight. Children weighing less than 20kg are given 1.875mg (1/2 dose); children weighing 20 - 30kg are given 2.5mg (2/3 dose); children weighing more than 30kg are given 3.75mg.
• Thereafter, injections are given every 3 - 4 weeks, according to effect.
The treatment duration is monitored by your doctor
If you are given more GONAPEPTYL Depot than you should
It is not very likely that you will be given more GONAPEPTYL Depot than you should have received. If you have been given more GONAPEPTYL Depot than you should, talk to a doctor or pharmacist immediately.
If you stop using GONAPEPTYL Depot
Treatment with GONAPEPTYL Depot should only be discontinued under advice from your doctor. If you have any further questions for the use of this product, ask your doctor or pharmacist.
turn over A
Tear here
Tire following information is intended for healthcare professionals only:
INSTRUCTIONS FOR USE
1. Preparation
Instructions; for the physidan how to [rrepare the suspension. Since successful treatment depends upon correct preparation of thesespension, the following instructions must be strictly followed.
• Take the package of GONAPEPTYL Depot from the refrigerator.
• Remove the cap from the disposable syringe containing the powder. Keep upright to prevent spilling.
• Open the package with the connector without removing the connector.
• Screw hhe syringe containing the suetained releose microparticles on the connector in the package, then remove it.
• Serow the syringe containing the suspension agent tightly on the free end of the connector and ensure that it fits tightly.
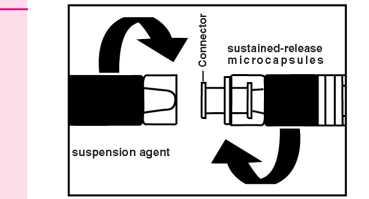
Preparation
turn over A
• pain
• headache
• blurred vision
• abdominal discomfort
• genital haemorrhage
• blood pressure increase
• weight gain
• Injection site pain, inflammation and redness
• general discomfort
• redness
• angioedema (swelling that occurs under the skin)
4. POSSIBLE SIDE EFFECTS
Like all medicines, GONAPEPTYL Depot can cause side effects, although not everybody gets them.
General (all patients):
If you experience swelling of the face, lips, mouth or throat which may cause difficulty in swallowing or breathing tell your doctor immediately or go to your nearest casualty department.
Cases of pre-existing pituitary tumour enlargement were reported during treatment with LH-RH agonists, however it has not yet been observed with triptorelin therapy.
In Men:
The symptoms you are being treated for (e.g. urinary obstruction, skeletal pain, compression of the spinal cord, muscular discomfort and oedema of the legs, weakness and tingling in the feet and hands) may worsen initially, due to the increased levels of testosterone at the start of treatment.
Very common, more than 1 patient out of every 10 patients treated: most of the side effects of GONAPEPTYL Depot in men result from lowered testosterone levels. Impotence, decreased libido, hot flushes, bone pain and difficulty and pain in passing urine can be seen.
Common, between 1 and 10 patients out of every 100 patients treated: allergic reaction, depressed mood, mood changes, depression, sleep disorder, nausea, muscle and joint pain, tiredness, injection site reaction, injection site pain, irritability, excessive sweating, headache and breast enlargement in males.
Uncommon, between 1 and 10 patients out of every 1000 patients treated: elevated values of some liver enzymes, anaphylactic reaction, testicular wasting, high blood pressure, decreased appetite, dry mouth, upper abdominal pain, asthma aggravated, weight changes, embolism, hair loss and reduced hair growth.
Not known, frequency cannot be estimated from the available data:
|
common cold |
• |
diabetes mellitus |
|
gout |
• |
abdominal bloating |
|
vertigo |
• |
constipation |
|
diarrhoea |
• |
shortness of breath |
|
injection site redness |
• |
influenza like symptoms |
|
sleepiness |
• |
blurred vision |
|
sensation of tingling, |
• |
nose bleeds |
|
pricking or numbness |
• |
memory impairment |
|
taste disturbances |
• |
visual impairment |
|
abnormal sensation in eye |
• |
tinnitus |
|
increased appetite |
• |
general discomfort |
|
anxiety |
• |
loss of libido |
|
insomnia |
• |
dizziness |
|
confusional state |
• |
chest pain |
|
decreased activity |
• |
chills |
|
fever |
• |
breast pain |
|
weakness |
r •i |
testicular pain |
|
ejaculation failure |
• |
joint swelling |
|
osteoarthritis |
• |
mnaeuloskeletal stiffness |
|
shortness of breach |
• |
injeetian site inflammation |
|
when iying flat |
• |
back pain |
|
jaoint stiffness |
• |
itching |
|
purzle discoloration of skin |
• |
pain in extremities |
|
musculoskoletal [3ain |
• |
muscular weakness |
|
flatulence |
• |
Sives |
|
Id lisrers |
• |
muscle apasms |
|
angioedoma iswelling that |
• |
difficulty in standing: |
|
srccuos under the skin) |
• |
oedema |
|
vomiting |
• |
acne |
|
abdominal °>ain |
• |
rash |
|
low bioop isressure |
• |
changes in ECG |
|
pain |
(QT psolongation) | |
|
euhhoric meod |
• |
increased bioot pressure |
|
body temperature increased |
• |
elevated values of some liver and kidney enzymes |
In Women:
Very common, mo re than 1 patient out of every 10 patients treated: decreased libido, mood changes , sleep) disorder, hot fluslies, abdominal pain, bone pain, excessive sweati ng, vaginal bleedi ng/spotting, vulvovaginal dryness, pa inful sexual i ntercourse, painful menstruation, enlargement of ovaries, pelvic paie, weakness and headachet
(OommoFi between 1 and 10 patients ouk ofevery 100 patients treated: allergic reaction, depressed mood depnession, nausea, muscle and joint pain, tiredness, injection sits reaction, injection site pain, irritability.
In Children:
Common, between 1 and 10 patients out of every 100 patients treated: mood changes, depression.
Uncommon, between 1 and 10 patients out of every 1000 patients treated: in girls vaginal bleeding or discharge may occur. Nausea, vomiting and anaphylactic reaction have been seen.
Not known, frequency cannot be estimated from the available data:
• allergic reactions
• nervousness
• abdominal pain
• visual impairment
• nose bleeds
• hot flushes
• rash
• hives
• muscle pain
• hair loss
• loosening or separation of growth zone of tubular bones
• emotional lability
Reporting of side effects
If you get any side effects, talk to your doctor. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme, website: www.mhra.gov.uk/yellowcard. By reporting side effects you can help provide more information on the safety of this medicine.
5. HOW TO STORE GONAPEPTYL DEPOT
Keep out of the reach and sight of children.
Do not use GONAPEPTYL Depot after the expiry date which is stated on the packaging. The expiry date refers to the last day of that month.
Store in a refrigerator (2 °C-8 °C)’. Keep the container in the outer carton.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. FURTHER INFORMATION
What GONAPEPTYL Depot contains
• The powder in each pre-filled syringe contains 4.12 mg of triptorelin acetate equivalent to 3.75 mg of the active substance, triptorelin.
• The other ingredients are Poly-(d,l lactide coglycolide), Propylene glycol dicaprylocaprate
Thesolvent contains
• Dextran 70, polysorbate 80, sodium chloride, sodium hydrogen phosphate dihydrate, sodium hydroxide and water for injection.
This medicinal product contains less than 1 mmol sodium (3.69 mg/ml or 0.160 mmol/ml) per dose, i.e it is essentially ‘sodium free’.
What GONAPEPTYL Depotlooks like and contents of the pack
It is presented in packs of 1 set of the following: 1 or 3 pairs of pre-filled syringes (powder and solvent).
Marketing authorisation holder:
Ferring Pharmaceuticals Ltd.
Drayton Hall, Church Road, West Drayton, UB7 7PS, UK
Manufacturer:
Ferring GmhH
Wittland 11, D-241 OS) Kiel, Germany
This medicinal product is authorised in the Member States of the EEA under the following names:
GO NAPEPTYL Depot (Belgium, Greece, Italy, Luxembourg, the Netherlands, Sweden, Spain, Portugal, United Kingdom),
UROPEPTYL Depot (Germany)
DECAPEPTYL GYN (Germany)
Uncommon, between 1 and 10 patients out of every 1000 patients treated: ananhylactic reaction, visnal impairment, sensaiio n of tingling, pric Oing or numbn ess, back pain, increased blood cholesterol, elevated values of some liver enzymes.
Not known, frequency cannot available data:
• abdominal discomfort
O heavy, prolonnod and/or irregular periods
• angioedema (swelling that occurs under the skin)
• loss of menstrual period
• loss of bone mineral
le ad ing t o increase d bone weaPness
• raslt
• anxiety
• diarrhoea
• vertigo
• blurred vision
• confusional state
be estimated from this
fever itc hing dizziness
injection site inflammat ion blood pressure increased breast pain muscle spasms weight changes l njection site rednees general discomfort vomiting
muscle weaknees hives
phortness of breath
GONAPEPTYL 3.75 mg (France)
GONAPEPTYL Depot 3.75 mg (Ireland)
DECAPEPTYn Depot (Czech Republic,
Denmark, Iceland, Estonia, Germany, Latvia, Lithuania, Norway, Poland, Slovakia)
DFCAPEPTYL Depol 3.75 (Finland)
DECAPEPTYL N (Germany)
GYNOPEPTYL (Germany)
DECAPEPTYL CR 3.75 (the Netherlands)
DECAPtPTYL Depot-Retardmikrokapseln und Suspensionsmittel fur Einmalspritzen (Austria)
DE CAPEPTYL Depot injection (Hungary)
DECAPEPTYL
Rhtart injectionspraparat
i.m/s.c (Switzerland).
This leaflet was last revised in August 2016.
Tear here
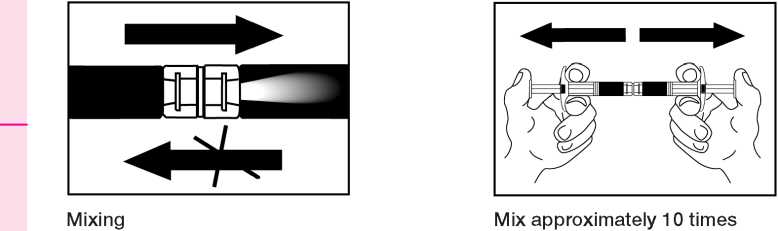
The following information is intended fsr healthcare professionals only:
2. Renonstitution of a sunpension
Empty the kquiS into tae syringe with the powdct, then shoet it back and forth into the first syringe - the first two or three times with out push ing tee injecti on rod all rice way in . Repeat this about 10 times or until you have a homogeneous milky white to faitrtly yellow seseension. Whils preparing the suspension, you might possibly create some foam. It is important that the foam lie dissolved or temoved from the syringe before giving the injection.
3. Ineection
• Remove the connsntor toget.er with the empty syringe.
• Msunt tae isjeciion nsedle on the syringe with the ready-to-use suspension.
• Inject subcutannously or deep into the muscle immediately.
GONAPEPTYL Dep>ot is for single nsa only and any unused suspension should be discarded.