Grazax 75 000 Sq-T Oral Lyophilisate
1059200 PIL UKIE GR 160121 GrazaxUKIE 21-01-2016 14:55 Sidel

1059200
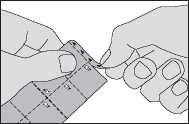
Tear a square off the oral lyophilisate pack along the perforated lines
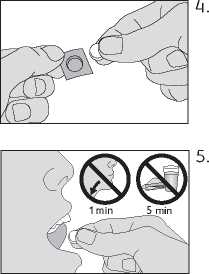
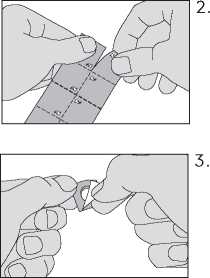
Remove the oral lyophilisate carefully from the foil and take the oral lyophilisate immediately
GRAZAX®
PACKAGE LEAFLET: INFORMATION FOR THE USER
GRAZAX® 75,000 SQ-T oral lyophilisate
Standardised allergen extract of grass pollen from Timothy (Phleum pratense)
Read all of this leaflet carefully before you start
taking this medicine.
- Keep this leaflet. You may need to read it again
- If you have further questions, ask your doctor or pharmacist
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours
- If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist
In this leaflet:
1. What Grazax is and what it is used for
2. Before you take Grazax
3. How to take Grazax
4. Possible side effects
5. How to store Grazax
6. Further information
1. What Grazax is and what it is used for
Grazax contains an allergen extract of grass pollen. Grazax is used to treat rhinitis and conjunctivitis caused by grass pollen in adults and children (5 years or older). Grazax modifies the allergic disease by increasing immunological tolerance towards grass pollen.
Children are carefully selected for treatment by doctors experienced in the treatment of allergic diseases in children.
The doctor will evaluate your allergic symptoms and make a skin prick test or take a blood sample in order to decide if Grazax should be used for treatment.
You are advised to take the first oral lyophilisate under medical supervision. This is a precaution in order to evaluate each patient's sensitivity to the treatment. This gives you the possibility of discussing possible side effects with the doctor.
Grazax is prescribed by doctors with experience in treatment of allergy.
2. Before you take Grazax
Do not take Grazax if:
- You are allergic (hypersensitive) to any of the excipients in the oral lyophilisate
- You have an illness which affects the immune system
- You have severe asthma (as assessed by your doctor)
- You have cancer
- You have a mouth inflammation which is severe
Take special care with Grazax if:
- You have recently had a tooth taken out or other forms of oral surgery. Treatment with Grazax should in this case be stopped for 7 days to allow your oral cavity to heal
- You have severe allergy to fish
- You previously have had an allergic reaction in connection with injection of allergen extract of grass pollen
Some side effects can be severe and need immediate medical care. Please see section 4 for symptoms.
Use in children
- Shedding of a deciduous (milk) tooth. Treatment with Grazax should in this case be stopped for
7 days to allow the oral cavity to heal
- In children with asthma experiencing an acute upper respiratory tract infection Grazax treatment should be temporarily discontinued until the infection has resolved.
If any of the above applies to you, talk to your doctor before taking Grazax.
There is no experience with Grazax in the elderly (65 years and older).
Taking other medicines
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription. If you are taking other medicines for your allergy symptoms such as antihistamines or corticosteroids your doctor should evaluate the use of such medicines.
Taking Grazax with food and drink
Food and drink should not be taken for 5 minutes after taking this oral lyophilisate.
Pregnancy and breast-feeding
At present there is no experience for the use of Grazax during pregnancy. Treatment with Grazax should not be initiated during pregnancy. If you become pregnant during treatment, speak to your doctor about whether it is appropriate for you to continue the treatment.
At present there is no experience for the use of Grazax during breast-feeding. No effects on the breastfed infants are anticipated.
Driving and using machines
You alone are responsible for the judgement of your ability to drive or perform precision work. Effects or side effects from medicine may influence this ability. A description of these effects is available in other sections of this leaflet. Thus, for guidance read all the information in this leaflet.
You should check with your doctor or pharmacist if you are not sure.
Treatment with Grazax has no or negligible influence on the ability to drive or use machines.
3. How to take Grazax
Always take Grazax exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
The usual dose is one oral lyophilisate daily. To get the best effect start taking the oral lyophilisates 4 months prior to the expected start of the grass pollen season. It is recommended to continue treatment for 3 years.
Use in children and adults
Grazax is an oral lyophilisate. Make sure your hands are dry before handling the oral lyophilisates. Take the oral lyophilisates like this:
1. Tear off the strip marked with triangles at the top of the oral lyophilisate pack.
Do not force the oral lyophilisate through the foil. It may damage the oral lyophilisate as it easily breaks. Instead, fold back the marked corner of the foil and then pull it off.
Place the oral lyophilisate under the tongue. Allow it to remain there for a few seconds until it has dissolved. Do not swallow during the first minute. Do not eat or drink for at least 5 minutes.
If you take more Grazax than you should
If you have taken too many Grazax oral lyophilisates you may experience allergic symptoms including local symptoms from mouth and throat. If you experience severe symptoms, immediately contact a doctor or a hospital.
If you forget to take Grazax
If you have forgotten to take an oral lyophilisate, take it later in the day. Do not take a double dose on any one day to make up for a forgotten oral lyophilisate.
If you stop taking Grazax
If you do not take this medicine as prescribed, you may not have an effect of the treatment. If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
21.01.2016 ALK Madrid
The side effects may be an allergic response to the allergen you are being treated with. In most cases the side effects last from minutes to hours after taking the oral lyophilisate and settles down within one week of starting the treatment.
Stop the intake of Grazax and contact your doctor or hospital immediately if you experience any of the following symptoms:
- Rapid swelling of face, mouth or throat
- Difficulties in swallowing
- Difficulties in breathing
- Hives
- Voice changes
- Worsening of existing asthma
- Severe discomfort
If you experience persisting heartburn you should contact your physician
Possible other side effects:
Very common (may affect more than 1 in 10 people):
- Common cold
- Mouth itching
- Irritating sensation in the throat Common (may affect up to 1 in 10 people):
- Headache
- Prickling sensation or numbness of the skin, mouth or tongue
- Eye or ear itching
- Eye, nose or mouth inflammation
- Asthma symptoms, shortness of breath, cough or sneezing
- Dry throat
- Nasal discomfort, stuffy or runny nose
- Swelling for example of lips or tongue
- Blistering or other discomfort of mouth, tongue or throat
- Stomach pain or discomfort, diarrhoea, feeling sick, vomiting
- Heartburn
- Itching in relation with for example rash, nettle rash or eczema
- Tiredness
- Chest discomfort or pain
- Fever
Uncommon (may affect up to 1 in 100 people):
- Sensation of rapid, forceful or irregular beating of the heart
- Lymph node swelling
- Dizziness
- Altered taste, decreased appetite
- Eye redness, irritation or swelling, tear flow
- Ear pain or discomfort
- Hoarseness
- Tightness, redness or numbness in the throat, painful swallowing
- Tonsil enlargement
- Allergic reaction
- Mouth redness or pain, dry mouth, swelling of roof of the mouth
- Lip blister, lip inflammation
- Salivary gland enlargement or hypersecretion
- Gum swelling or pain- Gastritis, regurgitation
- Feeling hot, feeling of discomfort
- Sensation of foreign body in the throat
- Skin redness, flushing
- Swelling of face or throat
Rare (may affect up to 1 in 1,000 people):
- Lower airways constriction
Eye irritation, ear pain, lip blister, salivary gland enlargement, throat redness, skin redness, allergic reaction and chest pain are reported more frequently in children than in adults.
If you have troublesome side effects you should contact your physician who will determine the anti-allergy medicines you may require, such as antihistamines.
Reporting of side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
You can also report side effects directly via the MHRA Yellow Card Scheme at www.mhra.gov.uk/yellowcard (UK) or the HPRA Pharmacovigilance Section at www.hpra.ie. E-mail: medsafety@hpra.ie (Ireland)
By reporting side effects you can help provide more information on safety of this medicine.
5. How to store Grazax
Keep out of the reach and sight of children.
Do not use Grazax after the expiry date stated on the blister and the carton after EXP. The expiry date refers to the last day of that month.
This medicinal product does not require any special storage conditions.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Further Information
What Grazax contains
The active substance is SQ standardised allergen extract of grass pollen from timothy (Phleum pratense). The activity per oral lyophilisate is expressed using the unit SQ-T*. The activity of one oral lyophilisate is 75,000 SQ-T.
* (Standardised Quality units Tablet (SQ-T)
The other ingredients are gelatine (fish source), mannitol and sodium hydroxide.
What Grazax looks like and contents of the pack
White to off-white circular oral lyophilisate marked with a debossed image on one side.
Aluminium blister cards with removable aluminium foil in an outer box of carton. Each blister card contains 10 oral lyophilisates.
Following packages are available: 30 (3x10),
90 (9x10) or 100 (10x10) oral lyophilisates.
Not all pack sizes may be marketed.
Marketing Authorisation Holder and Manufacturer
ALK-Abello A/S Boge Alle 6-8 DK-2970 Horsholm Denmark
This leaflet was last revised in July 2015
ALK
2325