Haemoctin 1000 Powder And Solvent For Solution For Injection

A Biotest
2. What you need to know before you use Haemoctin Do not use Haemoctin,
• if you are allergic to coagulation factor VIII or to any of the other ingredients of this medicine (listed in section 6). An allergic reaction may include rash, itching, difficulty breathing or swelling of the face, lips, throat or tongue.
Powder and solvent for solution for injection Human plasma derived coagulation factor VIII
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor, pharmacist or nurse.
• This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet:
1. What Haemoctin is and what it is used for
2. What you need to know before you use Haemoctin
3. How to use Haemoctin
4. Possible side-effects
5. How to store Haemoctin
6. Contents of the pack and other information
Warnings and precautions
If you start the treatment with Haemoctin, it is possible that your immune system develops antibodies (inhibitors) to factor VIII. These inhibitors may impact the effect of Haemoctin. Your doctor should check you regularly using a biological test (the Bethesda test) for the formation of inhibitors. The appearance of such factor VIII inhibitors manifests itself as a lack of therapeutic success. The amount of inhibitors in the body is expressed in Bethesda units (BU) per ml of blood plasma. The risk of developing inhibitors depends on the administration of factor VIII, and is greatest during the first 20 days of administration. Inhibitors rarely form after more than 100 days of administration. Cases of recurrent inhibitors have been observed after switching from one factor VIII product to another in previously treated patients with more than 100 exposure days who have a previous history of inhibitor development.
If you have existing cardiovascular risk factors, therapy with Haemoctin may increase the cardiovascular risk. If you are unsure, you should discuss this with your doctor.
Catheter-related complications: If a central venous access device (CVAD) is required, risk of CVAD-related complications including local infections, bacteraemia and catheter site thrombosis should be considered.
185391005
Haemoctin is a medicine derived from human plasma. It contains the coagulation factor VIII, which is necessary for a normal course of blood coagulation. After reconstitution of the powder with water for injections the solution is ready for intravenous injection.
Haemoctin is used for treatment and prophylaxis of bleeding in patients with haemophilia A (congenital factor VIII deficiency).
Haemoctin does not contain von Willebrand factor in pharmacologically effective quantities, and is therefore not suitable for the treatment of von Willebrand's disease.
Virus safety
When medicines are made from human blood or plasma, certain measures are put in place to prevent infections being passed on to patients. These include:
• careful selection of blood and plasma donors to make sure those at risk of carrying infections are excluded,
• the testing of each donation and pools of plasma for signs of virus/infections,
• the inclusion of steps in the processing of the blood or plasma that can inactivate or remove viruses.
Despite these measures, when medicines prepared from human blood or plasma are administered, the possibility of passing on infection cannot be totally excluded. This also applies to any unknown or emerging viruses or other types of infections.
The measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus and hepatitis C virus, and for the non-enveloped hepatitis A virus. The measures taken may be of limited value against non-enveloped viruses such as parvovirus B19.
Parvovirus B19 infection may be serious for pregnant women (fetal infection) and for individuals whose immune system is depressed or who have some types of anaemia (e.g. sickle cell disease or haemolytic anaemia).
Your doctor may recommend that you consider vaccination against hepatitis A and B if you regularly/repeatedly receive human plasma-derived Factor VIII products.
It is strongly recommended that every time you receive a dose of Haemoctin the name and batch number of the medicine are recorded in order to maintain a record of the batches used.
Children and adolescents:
The warnings and precautions for use mentioned for the adults should also be considered for children and adolescents.
Other medicines and Haemoctin
Tell your doctor if you are using, have recently used or might use any other medicines.
Interactions between Haemoctin and other medicinal products have not been reported.
Pregnancy, breast-feeding and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor for advice before taking this medicine.
Because of the rare occurrence of haemophilia A in women, there is no experience available on the use of factor VIII during pregnancy or while breast-feeding. No animal experiments have been performed during pregnancy and nursing.
Driving and using machines
Haemoctin has no or negligible influence on the ability to drive or use machines. Haemoctin contains sodium
Haemoctin 250: One vial contains up to 16.1 mg (0.70 mmol) sodium. Please take this into account if you are on a controlled sodium diet.
Haemoctin 500: One vial contains up to 32.2 mg (1.40 mmol) sodium. Please take this into account if you are on a controlled sodium diet.
Haemoctin 1000: One vial contains up to 32.2 mg (1.40 mmol) sodium. Please take this into account if you are on a controlled sodium diet.
Haemoctin is intended for intravenous administration (injection into a vein). Treatment should be under the supervision of a physician experienced in the treatment of haemophilia A. Always use Haemoctin exactly as your doctor told you. You should check with your doctor if you are not sure.
The dose and duration of treatment depend on the severity of the factor VIII deficiency, on the location and extent of the bleeding and on your clinical condition. Your doctor will determine the dose which is suitable for you.
Ensure sterile working in all steps of the procedure.

Fig. 1
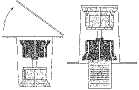
Fig. 2 Fig. 3

tasl .Si*
Fig. 4 Fig. 5

Fig. 6
Reconstitution of the powder:
• Bring the unopened vials of the solvent (water for injections) and product to room temperature. If a water bath is used for warming, it must be scrupulously ensured that the water does not come into contact with the caps or stoppers of the vials. Otherwise contamination of the medicine may occur.
• Remove the caps from both vials in order to expose the central portions of the rubber stoppers (1). Ensure that the rubber stoppers of the product and solvent vials are treated with a disinfectant.
• Remove the top of the transfer system packaging (2)
Place the blue part of the transfer system onto the upright standing vial containing the solvent (3).
• Remove the remaining part of the packaging of the transfer system. Now the transparent part of the transfer system is visible.
• Place the product vial on an even surface.
• Turn the combination of transfer system and solvent vial upside down. Push the spike of the transparent part of the adapter straight down through the product vial stopper (4). The vacuum present in the product vial causes the solvent to flow into the product vial. (5) Immediately unscrew the blue part of the transfer system together with the solvent vial. Discard the solvent vial with the blue part of the transfer system attached (6). Gently swirling the product vial helps in dissolving the powder. Do not shake vigorously, all foaming is to be avoided! The solution is clear or slightly opalescent.
• The solution ready for use should be used immediately after dissolving. Do not use solutions that are cloudy or contain visible particles.
| The following information is intended for healthcare professionals only:
Treatment monitoring
During the course of treatment, appropriate determination of factor VIII levels is advised to guide the dose to be administered and the frequency of repeated infusions. Individual patients may vary in their response to factor VIII, demonstrating different half-lives and recoveries. Dose based on bodyweight may require adjustment in underweight or overweight patients. In the case of major surgical interventions in particular, precise monitoring of the substitution therapy by means of coagulation analysis (plasma factor VIII activity) is indispensable. When using an in vitro thromboplastin time (aPTT)-based one stage clotting assay for determining factor VIII activity in patients' blood samples, plasma factor VIII activity results can be significantly affected by both the type of aPTT reagent and the reference standard used in the assay. Also there can be significant discrepancies between assay results obtained by aPTT-based one stage clotting assay and the chromogenic assay according to Ph. Eur. This is of importance particularly when changing the laboratory and/or reagents used in the assay.
Posology
The dose and duration of the substitution therapy depend on the severity of the factor VIII deficiency, on the location and extent of the bleeding and on the patient's clinical condition.
The number of units of factor VIII administered is expressed in International Units (IU), which are related to the current WHO concentrate standard for factor VIII products. Factor VIII activity in plasma is expressed either as a percentage (relative to normal human plasma) or preferably in International Units (relative to an International Standard for factor VIII in plasma).
One International Unit (IU) of factor VIII activity is equivalent to that quantity of factor VIII in one ml of normal human plasma.
On demand treatment
The calculation of the required dose of factor VIII is based on the empirical finding that 1 International Unit (IU) factor VIII per kg body weight raises the plasma factor VIII activity by 1 % to 2 % of normal activity.
The required dose is determined using the following formula:
Required units = body weight (kg) x desired factor VIII rise (%) x 0.5 The amount to be administered and the frequency of administration should always be oriented to the clinical effectiveness in the individual case.
In the case of the following haemorrhagic events, the factor VIII activity should not fall below the given plasma activity level (in % of normal) in the corresponding period. The following table can be used to guide dosing in bleeding episodes and surgery:
The following information is intended for healthcare professionals only:
|
Degree of haemorrhage / Type of surgical procedure |
Factor VIII level required (%) |
Frequency of doses (hours) / Duration of therapy (days) |
|
Haemorrhage | ||
|
Early haemarthrosis, muscle bleeding or oral bleeding |
20 - 40 |
Repeat every 12 to 24 hours. At least 1 day, until the bleeding episode as indicated by pain is resolved or healing is achieved. |
|
More extensive haemarthrosis, muscle bleeding or haematoma |
30 - 60 |
Repeat every 12 to 24 hours for 3 - 4 days or more until pain and acute disability are resolved. |
|
Life threatening haemorrhages |
60 - 100 |
Repeat every 8 to 24 hours until threat is resolved. |
|
Surgery | ||
|
Minor surgery including tooth extraction |
30 - 60 |
Every 24 hours, at least 1 day, until healing is achieved. |
|
Major surgery |
80 - 100 (pre- and postoperative) |
Repeat every 8 to 24 hours until adequate wound healing, then therapy for at least another 7 days to maintain a factor VIII activity of 30 - 60%. |
Prophylaxis
For long term prophylaxis against bleeding in patients with severe haemophilia A, the usual doses are 20 to 40 IU of factor VIII per kg body weight at intervals of 2 to 3 days. In some cases, especially in younger patients, shorter dosage intervals or higher doses may be necessary.
Method of administration:
Intravenous use. It is recommended not to administer more than 2 - 3 ml per minute.
Only the supplied infusion set should be used because treatment failure can occur as a consequence of factor VIII adsorption to the internal surfaces of some infusion equipment.
Haemoctin must not be mixed with other medicinal products.
J.
IT
*
Fig. 7
4. Possible side effects
If you notice any of the following effects, tell your doctor immediately:
• reddening of the skin,
• burning and stinging at the injection site
• chills
• flushing
• headache,
• hives,
• hypotension,
• lethargy,
• nausea,
• restlessness
• tachycardia,
• tightness of the chest,
• tingling,
• vomiting,
• wheezing
Injection:
• Once you have dissolved the powder as described above, screw the enclosed syringe with its Luer-Lock connector onto the product vial with the transparent part of the transfer system. (7) This allows you to easily draw the dissolved drug into the syringe. A separate filter is not necessary because the transfer system has its own integral filter.
• Carefully disconnect the vial with the transparent part of the transfer system from the syringe. Use the enclosed butterfly needle and administer immediately by slow intravenous injection. It is recommended not to administer more than 2-3 ml/min.
• After the butterfly needle has been used, it can be made safe with the protective cap.
If you use more Haemoctin than you should
If you believe that you have been given too much Haemoctin, tell your doctor, who will decide about further treatment.
If you forget to use Haemoctin
In this case your doctor will decide whether a further treatment is necessary.
If you stop using Haemoctin
Do not stop using Haemoctin without consulting your doctor.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Like all medicines, this medicine can cause side effects, although not everybody gets them.
This can be an allergic or a serious allergic reaction (anaphylactic shock) or a hypersensitivity reaction.
The following other side effects have been observed very rarely (less than 1 user in 10.000)
• Anti factor VIII antibody positive: Patients with haemophilia A may develop neutralising antibodies (inhibitors) to factor VIII. If such inhibitors occur, the condition will manifest itself as an insufficient clinical response (e.g. bleeding). In such cases, it is recommended that a specialised haemophilia centre be contacted.
• exanthema, urticaria, erythema
Side effects in children and adolescents
Side effects in children are expected to be the same as in adults.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the national reporting system: Yellow Card Scheme,
Website: www.mhra.gov.uk/yellowcard.
By reporting side effects you can help provide more information on the safety of this medicine.
5. How to store Haemoctin
Keep this medicine out of the sight and reach of children.
Keep the vials in the outer carton in order to protect from light.
Do not store above 25°C. Do not freeze.
Do not use Haemoctin after the expiry date which is stated on the label of the vial and the carton.
Any unused product or waste material should be disposed of in accordance with local requirements. Do not throw away any medicines via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the pack and other information What Haemoctin contains
• The active substance is Human coagulation factor VIII
• The other ingredients are glycine, sodium chloride, sodium citrate and calcium chloride.
• The solvent vial contains water for injections.
What Haemoctin looks like and contents of the pack
Haemoctin is supplied as a freeze-dried powder (lyophilisate). Water for injections serves as solvent. The dissolved product is clear or slightly opalescent.
Haemoctin 250 contains 1 vial with 250 IU and 1 vial with 5 ml water for injections (50 IU/ml)
Haemoctin 500 contains 1 vial with 500 IU and 1 vial with 10 ml water for injections (50 IU/ml)
Haemoctin 1000 contains 1 vial with 1000 IU and 1 vial with 10 ml water for injections (100 IU/ml)
Each pack contains
• one disposable syringe
• one transfer system with integral filter
• one butterfly cannula
Marketing Authorisation Holder and Manufacturer
Biotest Pharma GmbH Landsteinerstrasse 5 63303 Dreieich Germany
Phone: +49 6103 801-0 Fax: +49 6103 801-150 mail@biotest.de
This leaflet was last revised in 08/2016.
0