Haemonine 500 Powder And Solvent For Solution For Injection
SUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
Haemonine® 500 Haemonine® 1000
Powder and solvent for solution for injection
2 QUALITATIVE AND QUANTITATIVE COMPOSITION
Human plasma derived coagulation factor IX;
Haemonine® is presented as a powder and solvent for solution for injection containing either 500 or 1000 IU human coagulation factor IX per vial.
When reconstituted with either 5 ml or 10 ml water for injections, Haemonine® contains approximately 100 IU/ml human coagulation factor IX.
The potency (IU) is determined using the European Pharmacopoeia one stage clotting test. The specific activity of Haemonine® is > 70 IU/mg protein.
Excipients with known effect: The reconstituted product contains 0.19 mmol - 0,245 mmol (4.37 mg - 5,63 mg) sodium per ml. For the full list of excipients, see section 6.1.
3 PHARMACEUTICAL FORM
Powder and solvent for solution for injection.
White powder and clear, colourless solvent for solution for injection.
After dissolving the powder in the provided water for injections, the Haemonine solution is clear or slightly opalescent without any visible particles (see section 6.6).
4 CLINICAL PARTICULARS
4.1 Therapeutic indications
Treatment and prophylaxis of bleeding in patients with haemophilia B (congenital factor IX deficiency).
Haemonine is indicated in adults, adolescents and children aged 6 years and older.
4.2 Posology and method of administration
Treatment should be under the supervision of a physician experienced in the treatment of haemophilia.
Treatment monitoring
During the course of treatment, appropriate determination of factor IX levels is advised to guide the dose to be administered and the frequency of repeated infusions. Individual patients may vary in their response to factor IX, demonstrating different half-lives and recoveries. Dose based on bodyweight may require adjustment in underweight or overweight patients. In the case of major surgical interventions in particular, precise monitoring of the substitution therapy by means of coagulation analysis (plasma factor IX activity) is indispensable.
Posology
Dose and duration of the substitution therapy depend on the severity of the factor IX deficiency, on the location and extent of the bleeding and on the patient's clinical condition.
The number of units of factor IX administered is expressed in International Units (IU), which are related to the current WHO standard for factor IX products. Factor IX activity in plasma is expressed either as a percentage (relative to normal human plasma) or in International Units (relative to an International Standard for factor IX in plasma).
One International Unit (IU) of factor IX activity is equivalent to that quantity of factor IX in one ml of normal human plasma.
On demand treatment
The calculation of the required dosage of factor IX is based on the empirical finding that 1 International Unit (IU) factor IX per kg body weight raises the plasma factor IX activity by 1-2 % of normal activity. The required dose is determined using the following formula:
Required units = body weight (kg) x desired factor IX rise (%) (IU/dl) x 0.8
The amount to be administered and the frequency of administration should always be oriented to the clinical effectiveness in the individual case.
In the case of the following haemorrhagic events, the factor IX activity should not fall below the given plasma activity level (in % of normal or in IU/dl) in the corresponding period. The following table can be used to guide dosing in bleeding episodes and surgery:
Degree of haemorrhage/ Factor IX level Frequency of doses Type of surgical required (%) (hours)/Duration of therapy
procedure (IU/dl) (days)
|
Haemorrhage Early haemarthrosis, muscle bleeding or oral bleeding |
20 - 40 |
Repeat every 24 hours. At least 1 day, until the bleeding episode as indicated by pain is resolved or healing is achieved. |
|
More extensive haemarthrosis, muscle bleeding or haematoma |
30 - 60 |
Repeat infusion every 24 hours for 3 - 4 days or more until pain and acute disability are resolved. |
|
Life threatening haemorrhages |
60 - 100 |
Repeat infusion every 8 to 24 hours until threat is resolved. |
|
Surgery Minor surgery including tooth extraction |
30 - 60 |
Every 24 hours, at least 1 day, until healing is achieved. |
|
Major surgery |
80 - 100 (pre- and postoperative) |
Repeat infusion every 8 to 24 hours until adequate wound healing, then therapy for at least another 7 days to maintain a factor IX activity of 30 to 60% (IU/dl). |
Prophylaxis
For long term prophylaxis against bleeding in patients with severe haemophilia B, the usual doses are 20 to 40 IU of factor IX per kilogram of body weight at intervals of 3 to 4 days. In some cases, especially in younger patients, shorter dosage intervals or higher doses may be necessary.
Paediatric population
There are insufficient data to recommend the use of Haemonine® in children less than 6 years of age.
Method of administration
Intravenous use.
For instructions on dilution of the medicinal product before administration, see section 6.6. It is recommended to not exceed a maximal infusion rate of 5 ml/min.
4.3 Contraindications
Hypersensitivity to the active substance or to any of the excipients listed in section
6.1 or to heparin.
4.4 Special warnings and precautions for use
Hypersensitivity
Allergic type hypersensitivity reactions are possible with Haemonine. The product contains traces of human proteins other than factor IX. If symptoms of hypersensitivity occur, patients should be advised to discontinue use of the medicinal product immediately and contact their physician. Patients should be informed of the early signs of hypersensitivity reactions including hives, generalised urticaria tightness of chest, wheezing, hypotension, and anaphylaxis.
In case of shock, standard medical treatment for shock should be implemented.
Inhibitors
After repeated treatment with human coagulation factor IX products, patients should be monitored for the development of neutralising antibodies (inhibitors) that should be quantified in Bethesda Units (BU) using appropriate biological testing.
There have been reports in the literature showing a correlation between the occurrence of a factor IX inhibitor and allergic reactions. Therefore, patients experiencing allergic reactions should be evaluated for the presence of an inhibitor. It should be noted that patients with factor IX inhibitors may be at an increased risk of anaphylaxis with subsequent challenge with factor IX.
Because of the risk of allergic reactions with factor IX products, the initial administrations of factor IX should, according to the treating physician's judgement, be performed under medical observation where proper medical care for allergic reactions could be provided.
Thromboembolism
Because of the potential risk of thrombotic complications, clinical surveillance for early signs of thrombotic and consumptive coagulopathy should be initiated with appropriate biological testing when administering this product to patients with liver disease, to patients post-operatively, to new-born infants, or to patients at risk of thrombotic phenomena or DIC. In each of these situations, the benefit of treatment with Haemonine should be weighed against the risk of these complications.
Due to potential additive or synergistic pharmacodynamic effects, coadministration of antifibrinolytic agents with anti-inhibitor coagulant complex or factor IX complex could increase the risk of thrombosis.
Cardiovascular events
In patients with existing cardiovascular risk factors, substitution therapy with FIX may increase the cardiovascular risk.
Catheter-related complications
If a central venous access device (CVAD) is required, risk of CVAD-related complications including local infections, bacteraemia and catheter site thrombosis should be considered.
Transmissible agents
Standard measures to prevent infections resulting from the use of medicinal products prepared from human blood or plasma include selection of donors, screening of individual donations and plasma pools for specific markers of infection and the inclusion of effective manufacturing steps for the inactivation/removal of viruses. Despite this, when medicinal products prepared from human blood or plasma are administered, the possibility of transmitting infective agents cannot be totally excluded. This also applies to unknown or emerging viruses and other pathogens.
The measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus (HBV) and hepatitis C virus (HCV), and for the non-enveloped hepatitis A virus (HAV).
The measures taken may be of limited value against non-enveloped viruses such as parvovirus B19. Parvovirus B19 infection may be serious for pregnant women (fetal infection) and for individuals with immunodeficiency or increased erythropoiesis (e.g. haemolytic anaemia).
Appropriate vaccination (hepatitis A and B) should be considered for patients in regular/repeated receipt of human plasma-derived factor IX products.
It is strongly recommended that every time that Haemonine® is administered to a patient, the name and batch number of the product are recorded in order to maintain a link between the patient and the batch of the product.
Paediatric population
The listed warnings and precautions apply both to adults and children aged 6 years and older (see also section 4.2).
This medicinal product contains a maximum of 4.9 mmol (113 mg) sodium per standard dose of 2000 IU. To be taken into consideration by patients on a controlled sodium diet.
4.5 Interaction with other medicinal products and other forms of interaction
No interaction studies have been performed. No interactions of human coagulation factor IX products with other medicinal products have been reported.
Paediatric population
The listed interactions apply both to adults and children aged 6 years and older (see also section 4.2).
4.6 Fertility, pregnancy and lactation
Fertility data are not available.
Animal reproduction studies have not been conducted with factor IX. Based on the rare occurrence of haemophilia B in women, experience regarding the use of factor IX during pregnancy and breast-feeding is not available. Therefore, factor IX should be used during pregnancy and lactation only if clearly indicated.
4.7 Effects on ability to drive and use machines
Haemonine has no or negligible influence on the ability to drive and use machines.
4.8 Undesirable effects
Summary of the safety profile
Hypersensitivity or allergic reactions (which may include angioedema, burning and stinging at the infusion site, chills, flushing, generalised urticaria, headache, hives, hypotension, lethargy, nausea, restlessness, tachycardia, tightness of the chest, tingling, vomiting, wheezing) have been observed rarely and may in some cases progress to severe anaphylaxis (including shock). In some cases, these reactions have progressed to severe anaphylaxis, and they have occurred in close temporal association with development of factor IX inhibitors (see also section 4.4).
Nephrotic syndrome has been reported following attempted immune tolerance induction in haemophilia B patients with factor IX inhibitors and a history of allergic reaction.
Haemonine may contain traces of heparin below the limit of quantitation (0.1 IU/ml) which may cause hypersensitivity reactions and reduced blood cell counts which may affect the blood clotting system. Patients with a history of heparin-induced allergic reactions should avoid the use of heparin-containing medicines.
Patients with haemophilia B may develop neutralising antibodies (inhibitors) to factor IX. If such inhibitors occur, the condition will manifest itself as an insufficient clinical response. In such cases, it is recommended that a specialised haemophilia centre be contacted.
There is a potential risk of thromboembolic episodes following the administration of factor IX products, with a higher risk for low purity preparations. The use of low purity factor IX products has been associated with instances of myocardial infarction, disseminated intravascular coagulation, venous thrombosis and pulmonary embolism. The use of high purity factor IX is rarely associated with such side effects.
For safety information with respect to transmissible agents, see section 4.4.
Tabulated list of adverse reactions
Frequencies have been evaluated according to the following convention:
|
Very common: |
>1/10 |
|
Common: |
>1/100 to <1/10 |
|
Uncommon: |
>1/1,000 to <1/100 |
|
Rare: |
>1/10,000 to <1/1,000 |
|
Very rare: |
<1/10,000 |
|
not known |
cannot be estimated from the available data |
Frequency of Adverse Drug Reactions (ADRs) in clinical studies with Haemonine (Frequencies are calculated per patients treated (n=36)):
|
MedDRA Standard System Organ Class |
Frequency |
Adverse reactions |
|
Immune system disorders |
very common* |
Hypersensitivity |
|
Psychiatric disorders |
common |
Anxiety |
|
Nervous system disorders |
common |
Hyperaesthesia |
|
Gastrointestinal disorders |
common |
Nausea |
|
Skin and subcutaneous tissue disorders |
common |
Dermatitis allergic, Urticaria |
|
Musculoskeletal and connective tissue disorders |
common |
Back pain |
|
Vascular disorders |
common |
Hot flush |
|
Respiratory, thoracic and mediastinal disorders |
common |
Dyspnoea |
|
General disorders and administration site conditions |
common |
Feeling cold, Injection site reaction (including e.g. pain and rash) |
|
Investigations |
not known** |
factor IX inhibition |
* Hypersensitivity can be allergic or non-allergic reactions. True allergic reactions are rare.
** Reported from post-marketing sources.
Description of selected adverse reactions
Factor IX inhibition
The development of inhibitory antibodies is a known complication in the management of individuals with haemophilia B. There is no experience with previously untreated patients (PUPs) so far.
During clinical development no factor IX inhibitor induction was observed in previously treated patients (PTPs, n=36) during 1,493 exposure days.
Paediatric population
Frequency, type and severity of adverse reactions in children aged 6 years and older are expected to be the same as in adults (see also section 4.2).
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme: website: www.mhra.gov.uk/yellowcard.
4.9 Overdose
No case of overdose has been reported.
5 PHARMACOLOGICAL PROPERTIES
5.1 Pharmacodynamic properties
Pharmacotherapeutic Group: antihemorrhagics: blood coagulation factor IX. ATC code: B02BD04.
Factor IX is a single chain glycoprotein with a molecular mass of about 68,000
Dalton. It is a vitamin-K dependent coagulation factor and it is synthesised in the liver. Factor IX is activated by factor XIa in the intrinsic coagulation pathway and by the factor VII/tissue factor complex in the extrinsic pathway. Activated factor IX, in combination with activated factor VIII, activates factor X. Activated factor X converts prothrombin into thrombin. Thrombin then converts fibrinogen into fibrin and a clot is formed. Haemophilia B is a sex-linked hereditary disorder of blood coagulation due to decreased levels of factor IX and results in profuse bleeding into joints, muscles or internal organs, either spontaneously or as a result of accidental or surgical trauma. By replacement therapy the plasma levels of factor IX are increased, thereby enabling a temporary correction of the factor deficiency and correction of the bleeding tendencies.
Paediatric population
There are insufficient data to recommend the use of Haemonine® in children less than 6 years of age.
5.2 Pharmacokinetic properties
A pharmacokinetic study with 13 patients yielded the following results:
Using a biphasic model the mean initial half-life was 2.2 ± 1.9 h at initial visit and 3.1 ± 2.9 h at month 3, respectively. The mean terminal half-life was calculated as 28.5 ± 12.1 h at initial visit and 30.1 ± 14.7 h at month 3. The incremental recovery of Haemonine® was 69.8 ± 21.6 % and 72.2 ± 22.2 % at initial visit and at month 3, respectively. This corresponded to an incremental recovery of 0.015 ± 0.005 IU/ml/IU/kg body weight at initial visit and of 0.016 ± 0.005 IU/ml/IU/kg body weight at month 3. Other pharmacokinetic parameters of Haemonine® are: Area under the curve (AUC): about 25 IU • h/ml; Mean residence time (MRT): about 33 h; Clearance: about 200 ml/h.
5.3 Preclinical safety data
The preparation contains exclusively human plasma derived proteins, namely high purity coagulation factor IX, which is identical with the endogenous factor IX.
Preclinical studies in an Ames test showed no mutagenic potential of the preparation.
Haemonine® was tested for abnormal toxicity and thrombogenic potential in different rabbit models. The results revealed no signs for toxicological or thrombogenic potential.
List of excipients
6.1
Powder:
arginine
lysine
sodium chloride sodium citrate
Solvent:
water for injections.
6.2 Incompatibilities
This medicinal product must not be mixed with other medicinal products.
Use only the provided injection sets because treatment failure can occur as a consequence of human coagulation factor IX adsorption to the internal surfaces of some injection equipment.
6.3 Shelf life
2 years
Use immediately after reconstitution.
6.4 Special precautions for storage
Do not store above 25°C.
Do not freeze.
Keep the vials in the outer carton in order to protect from light.
6.5 Nature and contents of container
1 package Haemonine® 500 contains:
1 vial with powder, glass type I (Ph.Eur.), closed with chlorobutyl rubber stopper, type I (Ph.Eur.)
1 vial with solvent (5 ml), glass type I (Ph.Eur.), closed with bromobutyl rubber stopper, type I (Ph.Eur.)
The pack also contains:
1 disposable syringe (5 ml), 1 double-filter transfer system, 1 butterfly cannula.
1 package Haemonine® 1000 contains:
1 vial with powder, glass type I (Ph.Eur.), closed with chlorobutyl rubber stopper, type I (Ph.Eur.)
1 vial with solvent (10 ml), glass type I (Ph.Eur.), closed with bromobutyl rubber stopper, type I (Ph.Eur.)
The pack also contains:
1 disposable syringe (10 ml), 1 double-filter transfer system, 1 butterfly cannula.
6.6 Special precautions for disposal
Absolute sterility is to be ensured in all steps of the procedure !

Fig. 1
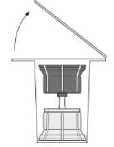
Fig. 2
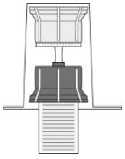
Fig. 3
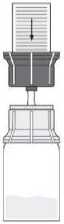
Fig. 5
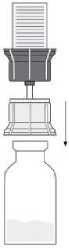
Fig. 4

Fig. 6
Dissolution of the concentrate:
• Bring the unopened vials of the solvent (water for injections) and product to room temperature. If a water bath is used for warming, it must be scrupulously ensured that the water does not come into contact with the caps or stoppers of the vials. Otherwise contamination of the medicine may occur.
• Remove the caps from both vials in order to expose the central portions of the rubber stoppers (1). Ensure that the rubber stoppers of the product and solvent vials are treated with a disinfectant.
• Remove the top of the transfer system packaging (2). Place the blue part of the transfer system onto the upright standing vial containing the solvent (3).
• Remove the remaining part of the packaging of the transfer system. Now the transparent part of the transfer system is visible.
• Place the product vial on an even surface.
• Turn the combination of transfer system and solvent vial upside down. Push the spike of the transparent part of the adapter straight down through the product vial stopper (4). The vacuum present in the product vial causes the solvent to flow into the product vial. (5) Immediately unscrew the blue part of the transfer system together with the solvent vial. Discard the solvent vial

Fig. 7
with the blue part of the transfer system attached (6). Gently swirling the product vial helps in dissolving the powder. Do not shake vigorously, all foaming is to be avoided! The solution is clear or slightly opalescent.
The solution ready for use must be used immediately after dissolving. Do not use solutions that are cloudy or have deposits.
Injection:
• Once you have dissolved the powder as described above, screw the enclosed syringe with its Luer-Lock connector onto the product vial with the transparent part of the transfer system. (7) This allows you to easily draw the dissolved drug into the syringe. A separate filter is not necessary because the transfer system has its own integral filter.
• Carefully disconnect the vial with the transparent part of the transfer system from the syringe. Use the enclosed butterfly needle and administer immediately by slow intravenous injection. The injection rate must not exceed 2-3 ml/minute.
• After the butterfly needle has been used, it can be made safe with the protective cap.
should be disposed of in accordance with local
Any unused product or waste material requirements.
7 MARKETING AUTHORISATION HOLDER
Biotest Pharma GmbH Landsteinerstrasse 5 63303 Dreieich Germany
Phone: +49 6103 801-0 Fax: +49 6103 801-150 mail@biotest.de
8 MARKETING AUTHORISATION NUMBER(S)
PL 04500/0008
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE AUTHORISATION
19/12/2008
10 DATE OF REVISION OF THE TEXT
28/06/2016