Haldol Decanoate 100Mg/Ml
Out of date information, search anotherSUMMARY OF PRODUCT CHARACTERISTICS
1 NAME OF THE MEDICINAL PRODUCT
HALDOL decanoate 100 mg/ml
2. QUALITATIVE AND QUANTITATIVE COMPOSITION
Haloperidol decanoate 141.04 mg, equivalent to 100 mg haloperidol base, per millilitre.
3. PHARMACEUTICAL FORM
Straw-coloured viscous solution for intramuscular injection.
4. CLINICAL PARTICULARS
4.1. Therapeutic Indications
Haldol decanoate is indicated for long term maintenance treatment where a neuroleptic is required; for example in schizophrenia, other psychoses (especially paranoid), and other mental or behavioural problems where maintenance treatment is clearly indicated.
4.2 Posology and method of administration
By intramuscular administration.
Haldol decanoate is for use in adults only and has been formulated to provide one month’s therapy for most patients following a single deep intramuscular injection in the gluteal region. Haldol decanoate must not be administered intravenously.
Since individual response to neuroleptic drugs is variable, dosage should be individually determined and is best initiated and titrated under close clinical supervision.
The size of the initial dose will depend on both the severity of the symptomatology and the amount of oral medication required to maintain the patient before starting depot treatment. Haldol decanoate injection should be used at the minimum dose that is clinically effective.
An initial dose of 50 mg every four weeks is recommended, increasing if necessary by 50 mg increments to 300 mg every four weeks. If, for clinical reasons, two-weekly administration is preferred, these doses should be halved. In patients with severe symptomatology, or in those who require large oral doses as maintenance therapy, higher doses of Haldol decanoate will be required. However, clinical experience with Haldol decanoate at doses greater than 300 mg per month is limited.
Routine administration of volumes greater than 3 mls at any one injection site is not recommended as larger volumes of injection are uncomfortable for the patient.
Haldol decanoate should be administered by deep intramuscular injection using an appropriate needle, preferably 2-2.5 inches long, of at least 21 gauge. Local reactions and medication oozing from the injection site may be reduced by the use of a good injection technique, eg the ‘Z-track’ method. As with all oily injections, it is important to ensure, by aspiration before injection, that intravenous entry has not occurred.
For patients previously maintained on oral neuroleptics, an approximate guide to the starting dose of Haldol decanoate is as follows: 500 mg of chlorpromazine a day is equivalent to 100 mg of Haldol decanoate monthly. The approximate equivalence for transferring patients previously maintained on fluphenazine decanoate or flupenthixol decanoate is as follows: 25 mg of fluphenazine decanoate 2-weekly or 40 mg of flupenthixol decanoate 2weekly is equivalent to 100 mg of Haldol decanoate monthly. This dose should be adjusted to suit the individual patient’s response.
Use in elderly:
It is recommended to start with low doses, for example 12.5 mg - 25 mg every four weeks, only increasing the dose according to the individual patient’s response.
4.3 Contraindications
Comatose states, CNS depression, Parkinson’s disease, known hypersensitivity to haloperidol or its excipients (contains sesame oil), lesions of basal ganglia. In common with other neuroleptics, haloperidol has the potential to cause rare prolongation of the QT interval. Use of haloperidol is therefore contraindicated in patients with clinically significant cardiac disorders e.g. recent acute myocardial infarction, uncompensated heart failure, arrhythmias treated with class IA and III antiarrhythmic medicinal products, QTc interval prolongation, history of ventricular arrhythmia or Torsades de pointes clinically significant bradycardia, second or third degree heart block and uncorrected hypokalaemia. Haloperidol should not be used concomitantly with other QT prolonging drugs (see section 4.5, Interactions).
4.4 Special warnings and precautions for use
Cases of sudden death have been reported in psychiatric patients receiving antipsychotic drugs, including haloperidol.
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10 week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality.
The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear.
Haldol Decanoate is not licensed for the treatment of dementia-related behavioural disturbances.
Cardiovascular effects
Very rare reports of QT prolongation and/or ventricular arrhythmias, in addition to rare reports of sudden death, have been reported with haloperidol. They may occur more frequently with high doses and in predisposed patients.
The risk-benefit of haloperidol treatment should be fully assessed before treatment is commenced and patients with risk factors for ventricular arrhythmias such as cardiac disease, family history of sudden death and/or QT prolongation, uncorrected electrolyte disturbances, subarachnoid haemorrhage, starvation or alcohol abuse, should be monitored carefully (ECGs and potassium levels), particularly during the initial phase of treatment, to obtain steady plasma levels.
The risk of QT prolongation and/or ventricular arrhythmias may be increased with higher doses (see Sections 4.8, and 4.9) or with parenteral use, particularly intravenous administration.
Haldol Decanoate must not be administered intravenously.
Haloperidol should be used with caution in patients known to be slow metabolisers of CYP2D6, and during use of cytochrome P450 inhibitors. Concomitant use of antipsychotics should be avoided. (See Section 4.5)
Baseline ECG is recommended prior to treatment in all patients, especially in the elderly and patients with a positive personal or family history of cardiac disease or abnormal findings on cardiac clinical examination. During therapy, the need for ECG monitoring (e.g. at dose escalation) should be assessed on an individual basis. Whilst on therapy, the dose should be reduced if QT is prolonged, and haloperidol should be discontinued if the QTc exceeds 500 ms. Periodic electrolyte monitoring is recommended, especially for patients taking diuretics, or during intercurrent illness.
An approximately 3-fold increase risk of cerebrovascular adverse events have been seen in randomised placebo controlled clinical trials in the dementia population with some atypical antipsychotics. The mechanism for this increased risk is not known. An increased risk cannot be excluded for other antipsychotics or other patient populations. Haloperidol should be used with caution in patients with risk factors for stroke.
Neuroleptic malignant syndrome
In common with other antipsychotic drugs, Haldol Decanoate has been associated with neuroleptic malignant syndrome: a rare idiosyncratic response characterised by hyperthermia, generalised muscle rigidity, autonomic instability, altered consciousness. Hyperthermia is often an early sign of this syndrome. Antipsychotic treatment should be withdrawn immediately and appropriate supportive therapy and careful monitoring instituted.
Tardive dyskinesia
As with all antipsychotic agents, tardive dyskinesia may appear in some patients on long-term therapy or after drug discontinuation. The syndrome is mainly characterised by rhythmic involuntary movements of the tongue, face, mouth or jaw. The manifestations may be permanent in some patients. The syndrome may be masked when treatment is reinstituted, when the dosage is increased or when a switch is made to a different antipsychotic drug. Treatment should be discontinued as soon as possible.
Extrapyramidal symptoms
In common with all neuroleptics, extrapyramidal symptoms may occur, e.g. tremor, rigidity, hypersalivation, bradykinesia, akathisia, acute dystonia. Antiparkinson drugs of the anticholinergic type may be prescribed as required, but should not be prescribed routinely as a preventive measure. If concomitant antiparkinson medication is required, it may have to be continued after stopping Haldol Decanoate if its excretion is faster than that of Haldol in order to avoid the development or aggravation of extrapyramidal symptoms. The physician should keep in mind the possible increase in intraocular pressure when anticholinergic drugs, including antiparkinson agents, are administered concomitantly with Haldol Decanoate.
Seizures/Convulsions
It has been reported that seizures can be triggered by Haldol Decanoate. Caution is advised in patients suffering from epilepsy and in conditions predisposing to convulsions (e.g., alcohol withdrawal and brain damage).
Hepatobiliary concerns
As Haldol Decanoate is metabolised by the liver, caution is advised in patients with liver disease. Isolated cases of liver function abnormalities or hepatitis, most often cholestatic, have been reported.
Endocrine system concerns
Thyroxine may facilitate Haldol Decanoate toxicity. Therefore, it should only be used with great caution in patients with hyperthyroidism. Antipsychotic therapy in those patients with hyperthyroidism should be used only with great caution and must always be accompanied by therapy to achieve a euthyroid state.
Hormonal effects of antipsychotic neuroleptic drugs include hyperprolactinaemia, which may cause galactorrhoea, gynaecomastia and oligo- or amenorrhoea. Very rare cases of hypoglycaemia and of Syndrome of Inappropriate ADH Secretion have been reported.
Venous thromboembolism
Cases of venous thromboembolism (VTE) have been reported with antipsychotic drugs. Since patients treated with antipsychotics often present with acquired risk factors for VTE, all possible risk factors for VTE should be identified before and during treatment with Haldol Decanoate and preventive measures undertaken.
Additional considerations
It is recommended that patients being considered for Haldol Decanoate therapy be initially put on oral haloperidol to exclude the possibility of an unexpected adverse sensitivity to haloperidol.
As with all antipsychotic agents, Haldol Decanoate should not be used alone where depression is predominant. It may be combined with antidepressants to treat those conditions in which depression and psychosis coexist. Haloperidol may impair the metabolism of tricyclic antidepressants (clinical significance unknown).
In schizophrenia, the response to antipsychotic drug treatment may be delayed. If drugs are withdrawn, recurrence of symptoms may not become apparent for several weeks or months.
Caution is advised in patients with renal failure and phaeochromocytoma
4.5. Interactions with other Medicinal Products and other forms of Interaction
Concomitant use of haloperidol with drugs known to prolong the QT interval may increase the risk of ventricular arrhythmias, including torsade de pointes. Therefore concomitant use of these products is not recommended (see section 4.3-Contraindications).
Examples include certain antiarrhythmics, such as those of Class 1A (such as quinidine, disopyramide and procainamide) and class III (such as amiodarone, sotalol and dofetilide), certain antimicrobials (sparfloxacin, moxifloxacin, erythromycin IV), tricyclic antidepressants (such as amitriptyline), certain tetracyclic antidepressants (such as maprotiline), other neuroleptics (e.g. phenothiazines, pimozide and sertindole), certain antihistamines (such as terfenadine), cisapride, bretylium and certain antimalarials such as quinine and mefloquine. This list is not comprehensive.
Concurrent use of drugs causing electrolyte imbalance may increase the risk of ventricular arrhythmias and is not recommended (see section 4.4-Special
Warnings and Precautions for Use). Diuretics, in particular those causing hypokalaemia, should be avoided but, if necessary, potassium-sparing diuretics are preferred.
Haloperidol is metabolised by several routes, including glucuronidation and the cytochrome P450 enzyme system (particularly CYP 3A4 or CYP 2D6). Inhibition of these routes of metabolism by another drug or a decrease in CYP 2D6 enzyme activity may result in increased haloperidol concentrations and an increased risk of adverse events, including QT-prolongation. In pharmacokinetic studies, mild to moderately increased haloperidol concentrations have been reported when haloperidol was given concomitantly with drugs characterised as substrates or inhibitors of CYP 3A4 or CYP 2D6 isozymes, such as, itraconazole, buspirone, venlafaxine, alprazolam, fluvoxamine, quinidine, fluoxetine, sertraline, chlorpromazine, and promethazine. A decrease in CYP2D6 enzyme activity may result in increased haloperidol concentrations. Increases in QTc and extrapyramidal symptoms have been observed when haloperidol was given with a combination of the metabolic inhibitors ketoconazole (400 mg/day) and paroxetine (20 mg/day). It may be necessary to reduce the haloperidol dosage.
Effect of Other Drugs on Haloperidol
When prolonged treatment with enzyme-inducing drugs such as carbamazepine, phenobarbital, rifampicin is added to Haldol Decanoate therapy, this results in a significant reduction of haloperidol plasma levels. Therefore, during combination treatment, the Haldol Decanoate dose or the dosage interval should be adjusted, when necessary. After stopping such drugs, it may be necessary to reduce the dosage of Haldol Decanoate.
Sodium valproate, a drug known to inhibit glucuronidation, does not affect haloperidol plasma concentrations.
Effect of Haloperidol on Other Drugs
In common with all neuroleptics, Haldol Decanoate can increase the central nervous system depression produced by other CNS-depressant drugs, including alcohol, hypnotics, sedatives or strong analgesics. An enhanced CNS effect, when combined with methyldopa, has been reported.
Haldol Decanoate may antagonise the action of adrenaline and other sympathomimetic agents and reverse the blood-pressure lowering effects of adrenergic blocking agents such as guanethidine.
Haldol Decanoate may impair the antiparkinsonian effects of levodopa.
Haloperidol is an inhibitor of CYP 2D6. Haldol Decanoate inhibits the metabolism of tricyclic antidepressants, thereby increasing plasma levels of these drugs.
Other Forms of Interaction
In rare cases, an encephalopathy-like syndrome has been reported in combination with lithium and Haldol decanoate. It remains controversial whether these cases represent a distinct clinical entity or whether they are in fact cases of NMS and/or lithium toxicity. Signs of encephalopathy-like syndrome include confusion, disorientation, headache, disturbances of balance and drowsiness. One report showing symptomless EEG abnormalities on the combination has suggested that EEG monitoring might be advisable. When lithium and haloperidol therapy are used concomitantly, haloperidol should be given in the lowest effective dosage and lithium levels should be monitored and kept below 1 mmol/l. If symptoms of encephalopathy-like syndrome occur, therapy should be stopped immediately
Antagonism of the effect of the anticoagulant phenindione has been reported.
The dosage of anticonvulsants may need to be increased to take account of the lowered seizure threshold.
4.6 Fertility, pregnancy and lactation
The safety of haloperidol in pregnancy has not been established. There is some evidence of harmful effects in some, but not all, animal studies. Neonates exposed to antipsychotic drugs (including haloperidol) during the third trimester of pregnancy are at risk of adverse effects including extrapyramidal and/or withdrawal symptoms that may vary in severity and duration following delivery. There have been reports of agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress, or feeding disorder. Consequently, newborns should be monitored carefully.
There have been a number of reports of birth defects following foetal exposure to haloperidol for which a causal role for haloperidol cannot be excluded.. Haldol decanoate should be used during pregnancy only if the anticipated benefit outweighs the risk and the administered dose and duration of treatment should be as low and as short as possible.
Haloperidol is excreted in breast milk. There have been isolated cases of extrapyramidal symptoms in breast-fed children. If the use of Haldol decanoate is essential, the benefits of breast feeding should be balanced against its potential risks.
4.7. Effects on Ability to Drive and Use Machines
Some degree of sedation or impairment of alertness may occur, particularly with higher doses and at the start of treatment, and may be potentiated by alcohol or other CNS depressants. Patients should be advised not to undertake activities requiring alertness such as driving or operating machinery during treatment, until their susceptibility is known.
4.8 Undesirable effects
The safety of Haldol Decanoate was evaluated in 410 subjects who participated in 3 comparator trials (one comparing haloperidol vs. fluphenazine and two comparing the decanoate formulation to the oral formulation), 9 open label trials and 1 dose responsive trial. The safety of Haldol was evaluated in 284 haloperidol-treated subjects who participated in 3 placebo-controlled, and in 1295 haloperidol-treated subjects who participated in sixteen double-blind active comparator-controlled clinical trials. Based on pooled safety data from these clinical trials, the most commonly reported (% incidence) Adverse Drug Reactions (ADRs) were: Extrapyramidal disorder (34), Insomnia (19), Agitation (15), Hyperkinesia (13), Headache (12).
Including the above mentioned ADRs, the following ADRs have been observed from clinical trials and post-marketing experiences reported with the use of Haldol and Haldol Decanoate.
Frequencies displayed use the following convention:Very common (> 1/10); common (> 1/100 to < 1/10); uncommon (> 1/1,000 to < 1/100); rare (> 1/10,000 to <1/1,000); very rare (<1/10,000), not known (cannot be estimated form the available data).
|
System Organ Class |
Adverse Drug Reactions | ||||
|
Frequency Category | |||||
|
Very Common (> 1/10) |
Common (> 1/100 to < 1/10) |
Uncommon (> 1/1,000 to < 1/100) |
Rare (> 1/10,000 to <1/1,000) |
Not Known | |
|
Blood and lymphatic System Disorders |
Leukopenia |
Agranulocytosis; Neutropenia; Pancytopenia; Thrombocytopenia | |||
|
Immune System Disorders |
Hypersensitivity |
Anaphylactic reaction | |||
|
Endocrine Disorders |
Hyperprolactinaemia |
Inappropriate Antidiuretic Hormone Secretion | |||
|
Metabolic and Nutritional Disorders |
Hypoglycaemia | ||||
|
Psychiatric Disorders |
Agitation; Insomnia |
Depression; Psychotic Disorder |
Confusional State; Libido Decreased; Loss of Libido; Restlessness | ||
|
Nervous System Disorders |
Extrapyramidal Disorder; Hyperkinesia; Headache |
Tardive dyskinesia; Dystonia; Dyskinesia; Akathisia; Bradykinesia; Hypokinesia; Hypertonia; Masked Facies; Somnolence; Tremor; Dizziness; |
Convulsion; Parkinsonism; Akinesia; Cogwheel Rigidity; Muscle Contractions Involuntary; Sedation |
Neuroleptic Malignant Syndrome; Motor Dysfunction; Nystagmus; | |
|
Eye Disorders |
Visual Disturbance; Oculogyric Crisis |
Vision Blurred | |||
|
Cardiac Disorders |
Tachycardia |
Ventricular fibrillation; Torsade de pointes; Ventricular tachycardia; Extrasystoles | |||
|
Vascular Disorders |
Orthostatic Hypotension; Hypotension | ||||
|
Respiratory, thoracic and mediastinal Disorders |
Dyspnoea |
Bronchospasm |
Laryngeal Oedema; Laryngospasm | ||
|
Gastro intestinal Disorders |
Constipation; Dry Mouth; Salivary Hypersecretion; Nausea; Vomiting | ||||
|
Hepatobiliary Disorders |
Liver function test abnormal |
Hepatitis; Jaundice |
Acute Hepatic Failure; Cholestasis | ||
|
Skin and subcutaneous tissue disorders |
Rash |
Photosensitivity reaction; Urticaria; Pruritis; Hyperhidrosis |
Leukocytoclastic Vasculitis; Dermatitis exfoliative | ||
|
Musculoskele tal and Connective Tissue Disorders |
Torticollis; Muscle rigidity; Muscle Spasms; Musculoskeletal stiffness |
Trismus; Muscle Twitching | |||
|
Renal and Urinary Disorders |
Urinary Retention | ||||
|
Pregnancy, Puerperium and Perinatal Conditions |
Drug withdrawal syndrome neonatal (see section 4.6) | ||||
|
Reproductive System and Breast Disorders |
Erectile Dysfunction |
Amenorrhoea; Dysmenorrhoea; Galactorrhoea; Breast pain; Breast discomfort |
Menorrhagia; Menstrual Disorder; Sexual Dysfunction |
Gynaecomastia, Priapism | |
|
General Disorders and Administratio n Site Conditions |
Injection Site Reaction |
Gait Disturbance; Hyperthermia; Oedema |
Sudden Death; Face Oedema; Hypothermia; Injection site abscess | ||
|
Investigations |
Weight Increased; Weight Decreased |
Electrocardiogram QT Prolonged |
Additional Information
Cardiac effects such as QT-interval prolongation, torsade de pointes, ventricular arrhythmias, including ventricular fibrillation and ventricular tachycardia, and cardiac arrest, have been reported. These effects may occur more frequently with high doses, and in predisposed patients
Toxic epidermal necrolysis and Stevens-Johnson syndrome have been reported in patients taking haloperidol. The true incidence of these reports is not known
Cases of venous thromboembolism, including cases of pulmonary embolism and cases of deep vein thrombosis have been reported with antipsychotic drugs- Frequency unknown.
4.9. Overdose
Symptoms
In general, the manifestations of haloperidol overdosage are an extension of its pharmacological actions, the most prominent of which would be severe extrapyramidal symptoms, hypotension and psychic indifference with a transition to sleep. The risk of ventricular arrhythmias possibly associated with QT-prolongation should be considered. The patient may appear comatose with respiratory depression and hypotension which could be severe enough to produce a shock-like state. Paradoxically, hypertension rather than hypotension may occur. Convulsions may also occur.
Treatment
There is no specific antidote to haloperidol. A patent airway should be established and maintained with mechanically assisted ventilation if necessary. In view of isolated reports of arrhythmia, ECG monitoring is strongly advised. Hypotension and circulatory collapse should be treated by plasma volume expansion and other appropriate measures. Adrenaline should not be used.
The patient should be monitored, body temperature and adequate fluid intake should be maintained.
In cases of severe extrapyramidal symptoms, appropriate anti-Parkinson medication should be administered.
5. PHARMACOLOGICAL PROPERTIES
5.1. Pharmacodynamic Properties
Pharmacotherapeutic group: Butyrophenone Derivatives:
ATC Code: NO5A DO1
The antipsychotic activity of haloperidol is principally due to its central dopamine blocking activity.
It has some activity against noradrenaline and less against serotonin. There is only very minimal activity against histamine and acetylcholine receptors.
5.2. Pharmacokinetic Properties
Haloperidol decanoate in solution is slowly released from the injection site and enters the systemic circulation, where it is hydrolysed by esterases to haloperidol. After an initial dose of 30-300 mg of haloperidol decanoate, plasma concentrations ranged from 0.8-3.2 ng/ml. After the second dose they were raised to 2.8 ng/ml which was steady state. A monthly dose of approximately 20 times the previous oral maintenance dose has been shown to be approximately clinically equivalent. Blood levels will vary considerably between patients.
5.3 Preclinical safety data
Only limited data are available, however these show no specific hazards apart from decreased fertility, limited teratogenicity as well as embryo-toxic effects in rodents.
Haloperidol has been shown to block the cardiac hERG channel in several published studies in vitro. In a number of in vivo studies intravenous administration of haloperidol in some animal models has caused significant QTc prolongation, at doses around 0.3 mg/kg i.v., giving Cmax plasma levels 3 to 7 times higher than the effective human plasma concentrations of 4 to 20ng/ml These intravenous doses which prolonged QTc did not cause arrhythmias. In some studies higher intravenous doses of 1 to 5 mg/kg haloperidol i.v. caused QTc prolongation and/or ventricular arrhythmias at Cmax plasma levels 19 to 68 times higher than the effective human plasma concentrations.
6. PHARMACEUTICAL PARTICULARS
6.1. List of Excipients
Benzyl alcohol Sesame oil
6.2. Incompatibilities
None known.
6.3. Shelf Life
36 months
Special Precautions for Storage
6.4.
Do not store above 25°C.
Do not refrigerate or freeze.
Keep ampoule in the outer carton to protect from light.
Lengthy storage in the cold may produce precipitation if this does not clear after further storage at room temperature, the contents of the ampoule should be discarded.
Keep out of reach and sight of children.
6.5. Nature and Content of Container
1 ml amber glass ampoule, in packs containing 5 ampoules.
6.6 Special precautions for disposal and other handling
Before use warm the ampoule in the hands to aid withdrawal of the contents.
1. Hold the body of the ampoule between the thumb and the index finger with the spot facing you.
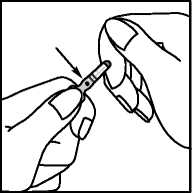
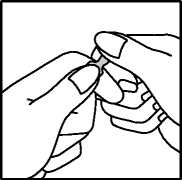
2. Position the index finger of the other hand so that it is supporting the neck of the ampoule. Position the thumb so that it covers the spot as shown below.
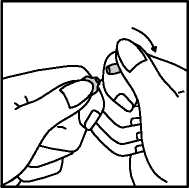
3. With the index fingers close together, apply firm downward pressure on the spot to snap the ampoule open.
7. Marketing Authorisation Holder
Janssen-Cilag Ltd
50-100 Holmers Farm Way
High Wycombe
Bucks
HP12 4EG
UK
8. MARKETING AUTHORISATION NUMBER
PL/0242/0095
9 DATE OF FIRST AUTHORISATION/RENEWAL OF THE
AUTHORISATION 23/06/2010
10 DATE OF REVISION OF THE TEXT
16/11/2011