Hapoctasin 52.5 Microgram/H Transdermal Patches
Hapoctasin 35microgram/h, 52.5microgram/h and 70microgram/h transdermal patches
Buprenorphine
Read all of this leaflet carefully before you start using this medicine because it
contains important information for you.
• Keep this leaflet. You may need to read it again.
• If you have any further questions, ask your doctor or pharmacist.
• This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
• If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
What is in this leaflet
j What Hapoctasin is and what it is used for
What you need to know before you use Hapoctasin How to use Hapoctasin Possible side effects How to store Hapoctasin q Contents of the pack and other information
j What Hapoctasin is and what it is used for
Hapoctasin is an analgesic (a pain-relieving medicine) intended to relieve moderate to severe cancer pain and severe pain that has not responded to other types of painkillers.
Hapoctasin acts through the skin. When the transdermal patch is applied to the skin, the active substance buprenorphine passes through the skin into the blood. Buprenorphine is an opioid (strong pain reliever), which reduces pain by acting on the central nervous system (specific nerve cells in the spinal cord and in the brain). The effect of the transdermal patch lasts for up to three days. Hapoctasin is not suitable for the treatment of acute (short-lasting) pain.
What you need to know before you use Hapoctasin Do not use Hapoctasin if you:
• are allergic to buprenorphine, soya, peanuts or any of the other ingredients of this medicine (listed in section 6)
• are dependent on strong pain relievers (opiods)
• suffer from a disease in which you have or may have great difficulty breathing
• are taking monoamine oxidase (MAO) inhibitors (certain medicines used to treat depression) or you have taken this type of medicines in the last two weeks (see "Taking other medicines")
• suffer from myasthenia gravis (a certain type of severe muscle weakness)
• suffer from delirium tremens (confusion and trembling caused by abstinence from alcohol following habitual excessive drinking or occurring during an episode of heavy alcohol consumption)
• if you are pregnant.
Hapoctasin must not be used to treat withdrawal symptoms in drug-dependent persons.
Warnings and precautions
Talk to your doctor or pharmacist before using Hapoctasin
• if you have recently drunk a lot of alcohol
• if you suffer from seizures or convulsions (fits)
• if your consciousness is disturbed (feeling light-headed or faint) for an unknown reason
• if you are in a state of shock (cold sweat might be a sign of it)
• if the pressure in your skull is increased (for instance after head injury or in brain disease), and artificial respiration is not possible
• if you have difficulty breathing or are taking other medicines that may make you breathe more slowly or weakly (see "Taking other medicines")
• if your liver does not work properly
• if you are inclined to abuse medicines or drugs.
Also, please be aware of the following precautions:
• Some people may become dependent on strong pain relievers such as Hapoctasin when they use them over a long period of time. They may have withdrawal effects when they stop using them (see "If you stop using Hapoctasin").
• Fever and external heat may lead to larger quantities of buprenorphine in the blood than normal. Also, external heat may prevent the transdermal patch from sticking properly. Therefore, do not expose yourself to external heat
(e.g. sauna, infra-red lamps, electric blankets, hot water bottles) and consult your doctor if you have fever. Hapoctasin should not be used in persons below the age of 18 years, because no experience has so far been gained in this age group.
Other medicines and Hapoctasin
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
• Hapoctasin must not be used together with monoamine oxidase (MAO) inhibitors (certain medicines used to treat depression), or if you have taken this type of medicine for the last 2 weeks.
• Hapoctasin may make some people feel drowsy, sick or faint or make them breathe more slowly or weakly. These side effects may be intensified if other medicines that may produce the same effects are taken at the same time. These other medicines include other strong pain relievers (opioids), certain sleeping pills, anaesthetics and medicines used to treat certain psychological diseases such as tranquillizers, anti-depressants and neuroleptics.
• If Hapoctasin is used together with some medicines, the effects of the transdermal patch may be increased. These medicines include e.g. certain anti-infectives/antifungals (e.g. containing erythromycin or ketoconazole) or HIV medicines (e.g. containing ritonavir).
• If Hapoctasin is used together with other medicines, the effects of the transdermal patch may be reduced. These medicines include certain products, e.g. dexamethasone; medicines to treat epilepsy (e.g. containing carbamazepine or phenytoin) or medicines for tuberculosis (e.g. rifampicin).
Hapoctasin with food, drink and alcohol
You should not drink alcohol while using Hapoctasin. Alcohol may intensify certain side effects of the transdermal patch and you may feel unwell. Drinking grapefruit juice may intensify the effects of Hapoctasin.
Pregnancy and Lactation
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Pregnancy
There is not sufficient experience regarding the use of Hapoctasin in pregnant women. Therefore you should not use Hapoctasin if you are pregnant.
Breast-feeding
Buprenorphine, the active substance contained in the transdermal patch, inhibits milk formation and passes into the breast milk. Therefore, you should not use Hapoctasin if you are breast-feeding.
Driving and using machines
Hapoctasin may make you feel dizzy or drowsy or experience blurred or double vision and affect your reactions to such an extent that you may not react adequately or quickly enough in the event of unexpected or sudden occurrences. This applies particularly
• at the beginning of treatment
• when your dosage is changed
• when you switch to Hapoctasin from another pain reliever
• if you also use other medicines that act on the brain
• if you drink alcohol.
If you are affected, you should not drive or operate machinery whilst using Hapoctasin. This applies also at the end of treatment with Hapoctasin. Do not drive or operate machinery for at least 24 hours after the patch has been removed.
Discuss with your doctor or pharmacist if you are unsure about anything.
The medicine can affect your ability to drive as it may make you sleepy or dizzy.
• Do not drive while taking this medicine until you know how it affects you.
• It is an offence to drive if this medicine affects your ability to drive.
• However, you would not be committing an offence if:
- The medicine has been prescribed to treat a medical or dental problem and
- You have taken it according to the instructions given by the prescriber or in the information provided with the medicine and
- It was not affecting your ability to drive safely
Talk to your doctor or pharmacist if you are not sure whether it is safe for you to drive while taking this medicine.
Hapoctasin contains soya oil.
If you are allergic to peanut or soya, do not use this medicinal product.
3| How to use Hapoctasin
Hapoctasin is available in three strengths:
Hapoctasin 35microgram/h transdermal patch, Hapoctasin 52.5microgram/h transdermal patch and Hapoctasin 70microgram/h transdermal patch.
The choice of which strength of Hapoctasin will suit you best will be made by your doctor. During treatment your doctor may change which transdermal patch you use to a smaller or larger one if necessary.
Always use Hapoctasin exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
The recommend dose is:
Adults
Unless your doctor has told you differently, attach one Hapoctasin transdermal patch (as described in detail below) and change it after 3 days at the latest. To help you remember when to change your transdermal patch, you should make a note on the outer packaging. If your doctor has advised you to take other pain relievers in addition to the transdermal patch, strictly follow the doctor's instructions, otherwise you will not fully benefit from treatment with Hapoctasin.
Patients under 18 years of age
Hapoctasin should not be used in persons below the age of 18 years, because no experience has so far been gained in this age group.
Elderly patients
No dosage adjustment is needed for elderly patients.
Patients with kidney disease / dialysis patients
In patients with kidney disease and in dialysis patients, no dosage adjustment is necessary.
Patients with liver disease
In patients with liver disease, the intensity and duration of action of Hapoctasin may be affected. If this applies to you, your doctor will check on you more closely.
Instruction for opening of child resistant pouch
1. Incise up to markings / arrowheads at each side
2. Rip at both notches along the heat-sealed joint
3. Open the pouch and take the patch
Method of administration
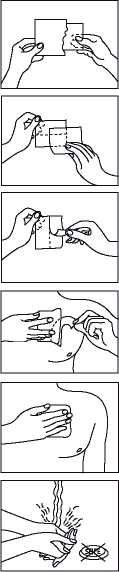
Before applying the transdermal patch
• Choose an area of skin which is flat, clean and hairless on your upper body, preferably on the chest below the collar-bone or on the upper part of the back. Call assistance if you cannot apply the transdermal patch yourself.
• If the chosen area has hairs, cut them off with a pair of scissors. Do not shave them off!
continued over page
3.
4.
5.
1.
2.
6.
• Avoid skin which is red, irritated or has any other blemishes, for instance large scars.
• The area of skin you choose must be dry and clean. If necessary, wash it with cold or lukewarm water. Do not use soap or other detergents. After a hot bath or shower, wait until your skin is completely dry and cool. Do not apply lotion, cream or ointment to the chosen area. This might prevent your transdermal patch from sticking properly.
Applying the transdermal patch:
Please do not open the sachet before you actually intend to use the transdermal patch. Each transdermal patch is sealed in a sachet.
Start with peel off the loose separation foil.
Peel off one half of the strip-foil of the transdermal patch and try not to touch the sticky part.
Stick the transdermal patch onto the area of skin you have chosen and remove the remaining foil.
Press the transdermal patch against your skin with the palm of your hand for 30 to 60 seconds. Make sure that the whole transdermal patch is in contact with your skin, especially at the edges.
Wash your hands after using the transdermal patch. Do not use any cleansing products.
Wearing the transdermal patch
You may wear the transdermal patch for up to 3 days. Provided that you have applied the transdermal patch correctly, there is little risk of it coming off.
You may shower, bathe or swim while wearing it. However, do not expose the transdermal patch to extreme heat (e.g. sauna baths, infra-red lamps, electric blankets, hot water bottles).
In the unlikely event that your transdermal patch falls off before it needs changing, do not use the same transdermal patch again. Stick a new one on straight away (see "Changing the transdermal patch" below).
Changing the transdermal patch
• Take the old transdermal patch off.
• Fold it in half with the sticky sides inwards.
• Throw it away carefully, out of the sight and reach of children.
• Stick a new transdermal patch on a different skin site (as described above). Wait at least one week before using the same skin site.
Duration of treatment
Your doctor will tell you how long you may use Hapoctasin. Do not stop using Hapoctasin on your own account, because pain may return and you may feel unwell (see also "If you stop using Hapoctasin" below).
If you have the impression that the effect of the Hapoctasin transdermal patch is too weak or too strong, tell your doctor or pharmacist.
If you use more Hapoctasin than you should
If this happens there may be signs of an overdose of the substance buprenorphine. An overdose may intensify the side effects of buprenorphine such as drowsiness, nausea and vomiting. You may get pin-point pupils and breathing may become slow and weak. You may also get cardiovascular collapse.
As soon as you discover that you have used more transdermal patch than you should, remove the excess transdermal patch and talk to a doctor or pharmacist.
If you forget to use Hapoctasin
If you forget an application, stick a new transdermal patch on as soon as you remember. If you are very late changing your transdermal patch, pain may return. In this case please contact your doctor.
Do not take a double dose to make up for the forgotten application.
If you stop using Hapoctasin
If you interrupt or finish using Hapoctasin too soon, pain may return.
If you wish to stop use on account of unpleasant side effects, please consult your doctor. He/she will tell you what can be done and whether you can be treated with other medicines.
Some people may experience withdrawal-effects when they have used strong pain relievers for a long time and stop using them. The risk of having effects after you stop using Hapoctasin is very low. However, if you feel agitated, anxious, nervous or shaky, if you are overactive, have difficulty sleeping or digestion problems, tell your doctor.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been reported:
Very common (may affect more than 1 in 10 people):
• nausea (feeling sick)
• redness, itching
Common (may affect up to 1 in 10 people):
• dizziness, headache
• shortness of breath
• vomiting, constipation
• skin changes (exanthema, generally on repeated use), sweating
• oedema (e.g. swelling of the legs), tiredness
Uncommon (may affect up to 1 in 100 people):
• confusion, sleep disorder, restlessness
• various degrees of sedation (calmness), ranging from tiredness to muzziness
• circulation disorders (such as low blood pressure or rarely, even circulatory collapse)
• dry mouth
• rash
• difficulty in passing water, urinary retention (less urine than normal)
• weariness
Rare (may affect up to 1 in 1,000 people):
• loss of appetite
• illusions such as hallucinations, anxiety and nightmares, reduced sex drive
• difficulty concentrating, speech disorder, muzziness, disturbed balance, abnormal skin sensations (numbness, prickling or burning sensations)
• visual disturbance, blurred vision, swollen eyelids
• hot flushes
• difficulty breathing (respiratory depression)
• heartburn
• hives
• erection difficulties
• withdrawal symptoms (see below), administration site reactions
Very rare (may affect up to 1 in 10,000 people):
• serious allergic reactions (see below)
• dependence, mood swings
• muscle twitching, taste disorders
• pin-point pupils
• ear pain
• abnormally rapid breathing, hiccups
• retching
• pustules, small blisters
• chest pain
In some cases delayed allergic reactions occurred with marked signs of inflammation. In such a case you should stop using Hapoctasin after you have talked to your doctor.
If you experience swelling of the hands, feet, ankles, face, lips, mouth or throat which may cause difficulty in swallowing and breathing, hives, fainting, yellowing of the skin and eyes (also called jaundice), remove the transdermal patch and call your doctor immediately or seek help at the casualty department of the nearest hospital. These can be symptoms of a very rare serious allergic reaction.
Some people may have withdrawal symptoms when they have used strong pain relievers for a long time and stop using them. The risk of having withdrawal effects when you stop using Hapoctasin is low. However, if you feel agitated, anxious, nervous or shaky, if you are overactive, have difficulty sleeping or digestion problems, tell your doctor.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard By reporting side effects you can help provide more information on the safety of this medicine.
How to store Hapoctasin
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and on the sachet after "Expiry date (month/year):". The expiry date refers to the last day of that month.
Storage conditions:
Do not store above 25°C.
Do not freeze.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6| Contents of the pack and other information What Hapoctasin contains
The active substance is: Buprenorphine.
Hapoctasin 35microgram/h transdermal patch: Each transdermal patch contains 20mg buprenorphine and releases about 35micrograms of buprenorphine per hour. The area of the transdermal patch containing the active substance is 25cm2. Hapoctasin 52.5microgram/h transdermal patch: Each transdermal patch contains 30mg buprenorphine and releases about 52.5micrograms per hour. The area of the transdermal patch containing the active substance is 37.5cm2.
Hapoctasin 70microgram/h transdermal patch: Each transdermal patch contains 40mg buprenorphine releases about 70micrograms per hour. The area of the transdermal patch containing the active substance is 50cm2.
The other ingredients are:
Drug containing adhesive matrix: styrene-butadiene-styrene (SBS) and styrene-butadiene block co-polymers, colophonium resin, antioxidants (2,4-Bis(1,1-Dimethylethyl)phenyl phosphite (3:1); Tris(2,4-Di-Tert-Butylphenyl)phosphate), aloe vera leaf extract oil (also contains refined soya-bean oil and alpha tocopherol acetate).
Backing foil: pigmented polyethylene, thermoplastic resin and aluminium vapour coated polyester, blue printing colour.
Release liner with pull off aid: polyester film, one side siliconised (to be removed prior application).
What Hapoctasin looks like and contents of the pack
The patches are tan coloured rectangular with four rounded edges and topped off corners and labelled with Hapoctasin 35 pg/h transdermal patch.
The patches are tan coloured, rectangular with four rounded edges and topped off corners and labeled with Hapoctasin 52.5 pg/h transdermal patch.
The patches are tan coloured, rectangular with four rounded edges and topped off corners and labeled with Hapoctasin 70 pg/h transdermal patch.
Each patch is packed in single sealed sachets.
Hapoctasin 35microgram/h is available in single sealed sachets of 4 transdermal patches.
Hapoctasin 52.5microgram/h is available in single sealed sachets of 4 transdermal patches.
Hapoctasin 70microgram/h is available in single sealed sachets of 4 transdermal patches.
The following strengths of the patches are available:
Hapoctasin 35microgram/h, Hapoctasin 52.5 microgram/h, Hapoctasin 70 microgram/h
Marketing Authorisation Holder
Actavis Group PTC ehf. Reykjavikurvegi 76-78, 220 HafnarfjorSur Iceland Manufacturers
Merckle GmbH, Ludwig-Merckle-Str. 3, 89143 Blaubeuren, Germany Actavis Group PTC ehf, Reykjavikurvegur 76-78, IS-220 Hafnarfjordur, Iceland Acino AG Am Windfeld 35 D-83714 Miesbach Germany
This leaflet was last revised in August 2015
If you would like a leaflet with larger text, please contact 01271 385257.
^actavis
Actavis, Barnstaple, EX32 8NS, UK
continued top of next column
AAAH8632